Read More
Read MoreSpecial Rules to Follow in Classifying Real Property or Land as an Ordinary Asset or Capital Asset and their Implications
This article delves into the special rules that govern the classification of real property or land as either an ordinary asset or a capital asset. Read More
Read MoreKnow When to Pay Value Added Tax or Capital Gains Tax When you Sell Real Property or Land
This article focuses on the crucial decision of when to pay either Value Added Tax (VAT) or the Capital Gains Tax on the sale of real property or land. Read More
Read MoreCan the Heirs of a Creditor file a Case to Collect a Debt?
This article answers whether the heirs of a creditor can collect a debt owed to their deceased relative. Read More
Read MoreImportant Rules to Follow for Validity of any Corporate Meeting
This article outlines important rules to follow to for validity of corporate meetings under the Revised Corporation Code of the Philippines Read More
Read MoreLACHES OR STALE DEMANDS: IF YOU SLEEP ON YOUR RIGHTS, YOU MAY LOSE THEM
This article discusses the doctrine of laches, or when there is an unjustifiable delay in invoking one’s rights. Read More
Read MoreHow Many Incorporators are Required to Form a Corporation?
The Revised Corporation Code allows one (1) incorporator for a One Person Corporation (OPC) while a corporation that is not an OPC requires at least two (2) but not more than fifteen (15) incorporators. Read More
Read MoreWhat is the Minimum Number of Shares of Stock to Form a Corporation?
There is NO minimum number of shares of stock required for a corporation to possess. However, certain industries or corporations with foreign equity are required to have a minimum paid-up capital. Read More
Read MoreWHEN CAN A COMPANY REFUSE TO GIVE CHRISTMAS BONUS?
This article explains an instance when a company can withdraw the grant of a Christmas bonus, even if the same has become customary or company practice. Read More
Read MoreThe Laws On Christmas In The Philippines
The Philippine legal system is a civil law system. This means that the Philippines relies on laws enacted by Congress to govern all matters – including Christmas, our favorite holiday. Christmas did not just become a holiday in the Philippines by tradition or religion. As with all red letter days and public holidays, there are laws that govern the celebration of Christmas as a holiday. Read More
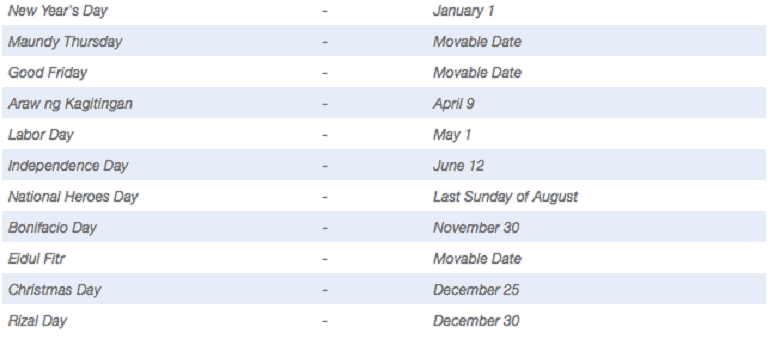
Read MoreLearn about Philippine Holidays and Special Non-Working Days for 2024
This article discusses the Philippine Regular and Special Holidays and Non-Working Days for the year 2024, as declared under Proclamation No. 368. Read More
Read MoreUnderstanding Property Ownership: The Legal Significance of the Phrase “Married to” in Transfer Certificates of Title
This article discusses the legal significance of the phrase “Married to” in Transfer Certificates of Title. Read More
Read MoreWhat Laws Govern Doctors and Medical Practitioners in the Philippines?
This article talks about the practice of medicine in the Philippines Read More
Read MoreIs a Property Donated during Marriage to only One Spouse Considered Conjugal Property?
This article answers whether property donated during marriage to one spouse is considered conjugal property. Read More
Read MoreHow to Recover Electronic or Digital Assets under Philippine Law
This article talks about how to recover electronic or digital assets under Philippine law. Read More
Read MoreHow does Philippine Law consider Illicit Common Law or De Facto Relationships?
This article discusses how Philippine Law considers illicit common law or de facto relationships Read More
Read MoreWhat are the Effects of a Confession of Judgment under Philippine Law
This article talks about the effects of a confession of judgment under Philippine law and procedure. Read More
Read MoreHow Can a Foreigner own a House or a Building in the Philippines?
This article talks about foreign ownership of real property, particularly of a house in the Philippines. Read More
Read MoreWhy Petitions for Naturalization are Dismissed?
This article explores the intricate process of judicial naturalization in the Philippines and delves into the key factors that often lead to the dismissal of such petitions, shedding light on the complexities and nuances of this significant legal journey. Read More
Read MoreCan a City Ban the Operation of Lotto and Casinos in its Territory?
This article talks about the police power of a local government unit such as city, including the limitations thereof. When lotto and casinos are allowed by the state, a city, through an ordinance, cannot ban the operations thereof in its territory. Read More
Read MoreBeing Loud Can Be a Crime!
This article talks about the crime of alarms and scandals such as discharging explosives, making loud noises, disturbing public peace and making a scandal in public punishable under the Revised Penal Code of the Philippines. Read More
Read MorePolice Power of the State
This article talks about the police power of the state and how it should be lawfully exercised by the government under the Philippine Constitution. Read More
Read MoreThe Crime of Physical Injuries under Philippine Law
This article talks about the crime of serious physical injuries, less serious physical injuries, slight physical injuries and maltreatment punishable under the Revised Penal Code of the Philippines. Read More
Read MoreREPUBLIC ACT 11595: LOWERING CAPITAL REQUIREMENTS FOR FOREIGN TRADE FIRMS
This article discusses the changes introduced by Republic Act 11595 to Republic Act 8762 also known as the Retail Trade Liberalization Act of 2000. Read More
Read MoreCRIMES INVOLVING CHILD MARRIAGES AND ILLICT RELATIONSHIPS WITH THEM
This article is about Republic Act 11596 which criminalizes and punishes child marriages and cohabitation with children.- Read More
Republic Act 11337 also known as the Innovative Startup Act
his article discusses Republic Act 11337, the law that supports startup companies and business in the Philippines  Read More
Read MorePhilippine Holidays 2023 and Special Non-Working Days
This article discusses the Philippine Holidays 2023 and Non-Working Days for the year 2023, as declared under Proclamation No. 90. Read More
Read MoreNew Law to Protect Consumers of Financial Products and Services
This article talks about Republic Act No. 11765, also known as "Financial Products and Services Consumer Protection Act”. This law aims to protect consumers of financial products and services. Read More
Read MoreStricter Requirements and Penalties for Contractors Imposed
This article talks about Republic Act No. 11711 which amended the old Contractor’s License Law, and increased the fines and penalties, clarified the validity of license to operate and granted longer renewal periods for contractors in good standing. Read More
Read MoreSolo Parents to Receive Additional Benefits
This article talks about Republic Act No. 11861 which expanded the rights and benefits of solo parents or single parents by introducing penalties for violation, creation of a system for registration for Solo Parents, and institutionalized the management of solo parents and benefits by creating a local government office designated to administer, monitor and regulate the identification and registration of solo parents. Read More
Read MoreWho has Custody over an Illegitimate Child if the Mother Passes Away?
This article answers the question as to who has custody over an illegitimate child after the mother dies. Read More
Read MoreWhen a Party is Non-Suited: The Importance and Consequences of Not Attending Pre-Trial
This article discusses the importance of pre-trial and the effects where a party fails to attend the pre-trial of a case. Read More
Read MoreWhat is the Tax Aspect of Closing a Branch or Department of a Company?
This article discusses the tax implications and requirements for closing a branch or department of a company. Read More
Read MoreWhat is a Proxy?
This article is all about corporate proxies, their use, and the laws and regulations that govern them in the Philippines. Read More
Read MoreThings You Ought to Know About Data Privacy in the Philippines
This article discusses the key points of the Philippines’ Data Privacy Act of 2012. Read More
Read MoreTaxing PAGCOR Licensees and Service Providers
This article discusses how PAGCOR licensees, service providers and suppliers are taxed. Read More
Read MoreHow to Close a Branch or Department in a Company
This article discusses how to close a department or branch of a company. Read More
Read MoreHow are Consultants Taxed?
This article discusses how consultants are taxed under Philippine laws. Read More
Read MoreCan you Be Entitled to Receive Separation Pay and Retirement Pay At the Same Time?
This article answers whether an employee can be entitled to separation pay and retirement pay simultaneously. Read More
Read MoreConsent in the Collection of Data: What Type of Consent Does the Data Privacy Law Require?
This article discusses about the different kinds of consent required under the Data Privacy Act, in the collection of data. Read More
Read MoreAre Cookie Banners required under Philippine Data Privacy Laws?
This article discusses whether cookie banners are required under the data privacy laws of the Philippines. Read More
Read MoreAbsorbing Employees in a Merger or Takeover
This article talks about the effect of mergers or acquisitions in the status of employment of the employees of the absorbed company. Read More
Read MoreNo Birth Certificate, No Problem! File an Application for Delayed Registration of Birth!
This article talks about the remedy of filing an application for delayed registration of birth with the local civil registry office where one has no birth certificate. Read More
Read MoreFailure to Give Child Support as Economic Abuse and Psychological Violence
This article talks about the liability of an accused in case of failure to give child support. This may take the form of either economic abuse or psychological violence under RA 9262 or VAWC. Read More
Read MoreHabitual Absenteeism as a Ground for Termination of Employment
This article talks about being fired from work or termination of employment due to habitual or numerous absences. Read More
Read MoreWho are the Heirs When Someone Dies Without a Will?
This article talks about the people who will inherit or are considered heirs when someone dies without a will. Read More
Read MoreFDA Suspends Product Registration for Household Hazardous Goods until 2024
This Article discusses the suspension of product registration requirements under FDA Circular No. 2021-011-A. Read More
Read MoreContinuing A Partnership Business After Partner Withdraws
This article discusses continuing a partnership business after a partner withdraws or leaves. Read More
Read MoreHow To Deal with a Work-Related Death in Your Company
This articles discusses how to deal with work-related death of employees in the company. Read More
Read MoreWithdrawal of a Partner Dissolves a Partnership
This article discusses the effect of withdrawal of a partner from a partnership or joint venture. Read More
Read MoreData Sharing in the Philippines
Republic Act No. 10173, otherwise known as the Data Privacy Act of 2012, protects and regulates the sharing or processing of personal information in the Philippines. Read More
Read MoreLearning about Electronic Money Issuers (EMI) in the Philippines
Electronic Money Issuers (EMIs) are regulated by the Bangko Sentral ng Pilipinas (BSP). Read More
Read MoreBringing in Foreign Investors in Retail Trade
Foreign investors who wish to engage in retail trade in the Philippines should have a minimum paid-up capital of Twenty-Five Million Pesos (P25,000,000). Read More
Read MoreLimitations on Foreign Investments and Equity in the Philippines
Foreign investments are encouraged by the Philippine government to fuel economic growth. Based on data culled from the National Statistical Coordination Board (“NSCB”), the total approved foreign investments in the Philippines for the year 2014 amounted to P186.9 Billion Pesos (or roughly US$4 Billion Dollars). According to the NSCB, in 2014, Japan is the largest […] Read More
Read MoreHow to Elect Directors of a Corporation
Directors are elected by the stockholders of a corporation in an election held where the owners of majority of the outstanding capital stock, whether in person or through proxy, are present. Read More
Read MoreWhat You Need to Know When Employing Night Workers
This article is about night workers. A night worker is one who performs no less than seven (7) consecutive hours of work within the period of 10 p.m. to 6 a.m. The law mandates health assessments, certain facilities and the protection of rights of night workers. Read More
Read MoreProtection of Trade Names
A trade name pertains to the identity of a company and is given protection by the law even prior to or without registration, against any unlawful act committed by third parties. The right to the exclusive use of a corporate name with freedom from infringement by similarity is determined by priority of adoption. The injured company can seek relief from the SEC. Read More
Read MoreLearn About the 2022 Philippine Holidays
This article discusses the Philippine Holidays, Special Working and Non-Working Days for the year 2022, as declared under Proclamation No. 1236. Read More
Read MoreUnderstanding Penalties in Criminal Laws in the Philippines
This article discusses penalties under criminal laws in the Philippines, the Revised Penal Code, and Special Penal Laws, and how to determine the imposable penalty. Read More
Read MoreKNOW THIS! HOW LAWS ARE PASSED IN THE PHILIPPINES
This article discusses how a law is passed in Philippines. Read More
Read MoreWho is a Nuisance Candidate?
This article defines a nuisance candidate as one who puts the election process in mockery, cause confusion among the voters and clearly demonstrate that the candidate has no bona fide intention to run for public office. The Comelec may, motu propio or upon a verified petition (filed within 5 days from the last day for the filing of certificate of candidacy), declare a candidate a nuisance. Read More
Read MoreNew Corporate Income, Reduced Taxes, and New Tax Incentives in the Philippines under RA 11534 (CREATE Act)
This article discusses the changes introduced by Republic Act No. 11534 or the CREATE Act on the corporate income tax, other taxes and on fiscal incentives in the Philippines.- Read More
Four Things You Need to Establish the Easement of Right of Way
This article talks about the elements and requisites to claim, demand and enforce the easement of right of way. - Read More
How do you Dissolve a Corporation in the Philippines?
This article sets out the procedure and requirements for dissolving and liquidating a Philippine corporation. - Read More
Why the Creation of a Creditors’ Committee is Beneficial for Unsecured Creditors
This article discusses why the creation of a creditors’ committee is beneficial for unsecured creditors in a rehabilitation proceeding under the Financial Rehabilitation and Insolvency Act of 2010.  Read More
Read MoreSteps Required to Declare Cash Dividends in Corporations
This outlines the steps which must be complied with for a corporation to validly declare cash dividends.- Read More
Are Christmas Breaks Given to Employees Considered Benefits?
This article talks about Christmas breaks and other employee benefits, and if these can be unilaterally withdrawn by the employee.  Read More
Read MoreHow to Implement a Redundancy Program during a Corporate Spin-Off
This article talks about implementing a redundancy program under a corporate spin-off. Read More
Read MoreArbitration as the Preferred Mode of Resolving Disputes in Government Procurement
This article arbitration as a mode of dispute resolution in government procurement and public bidding under Republic Act No. 9184. Read More
Read MoreThe Philippine Party-List System
This article talks about the Philippine party-list system, its composition, parameters and the method of computing the allocated number of seats. Read More
Read MoreCOVID-19 Labor Advisories from the Department of Labor and Employment
This Article discusses recent COVID-19 Labor Advisories Issued by the Department of Labor and Employment. Read More
Read MoreLowering the Height Requirement for Law Enforcers in the Philippines
This article discusses Republic Act No. 11549 also known as the PNP, BFP, BJMP and BuCor Height Equality Act, providing new height requirements for employment in the law enforcement service. Read More
Read MoreDrastic Changes in the Jurisdiction of Courts in Civil Cases
This article discusses Republic Act No. 11576, which changes the jurisdiction of the trial courts in the Philippines. Read More
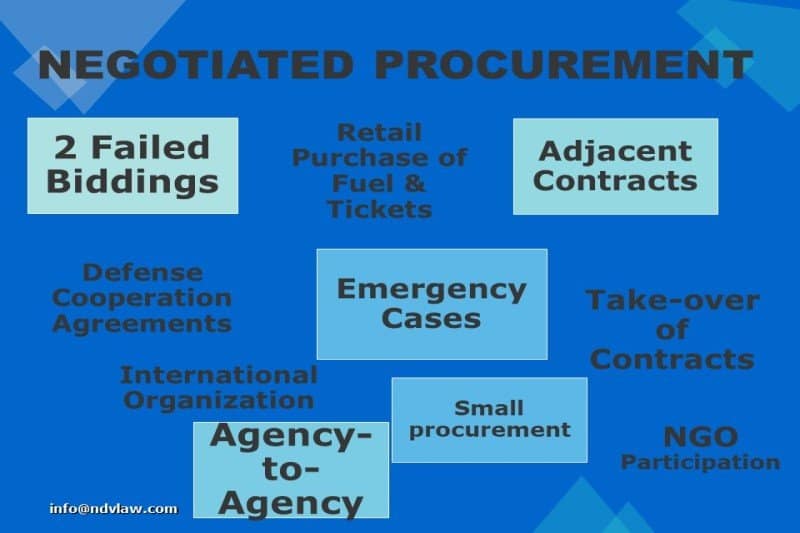
Read MoreWhat is Negotiated Procurement?
This article talks about negotiated procurement or a method of procurement resorted to under extraordinary circumstances whereby the government directly negotiates a contract with a technically, legally and financially capable supplier, contractor or consultant. Negotiated procurement is allowed in cases of two failed biddings, emergency cases, take-over of contracts, adjacent or contiguous contracts, agency-to-agency, exclusive technology services, highly technical consultants, defense cooperation agreements, small value procurement, lease of venue for official use, NGO participation, community participation, international organizations and direct retail purchase of fuel and airline tickets. Read More
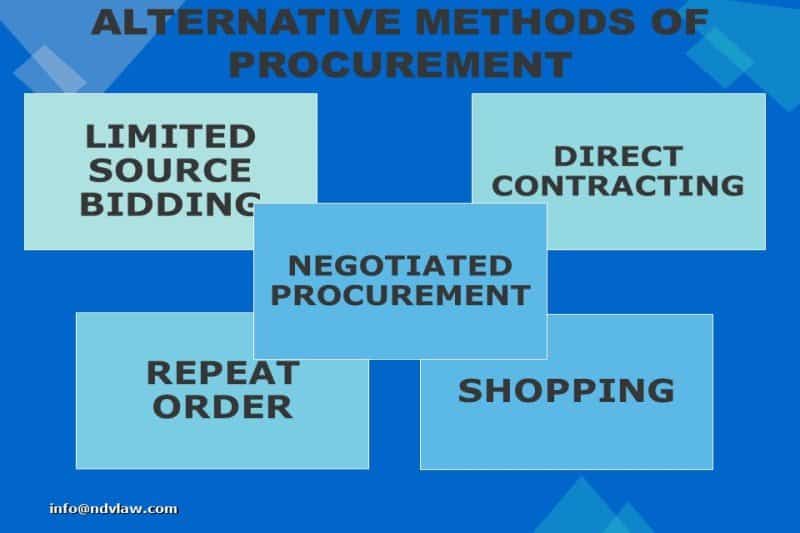
Read MoreExceptions to Public Bidding in Government Procurement
This article talks about the exceptions to public bidding in government procurement. This pertains to alternative methods of government procurement such as limited source bidding, direct contracting, repeat order, shopping and negotiated procurement. Read More
Read MoreWhat Happens to Government Officials Who File their Certificates of Candidacy?
Appointive government officials who file their Certificates of Candidacy are considered as deemed resigned while incumbent elective officials may run for an elective position without forfeiting their seats. Read More
Read MoreWhat is the Right Against Self-Incrimination?
The right against self-incrimination includes the right to refuse to take the witness stand and the right to refuse to answer an incriminatory question. This article talks about the constitutional right against self-incrimination, who can use it and when it can be invoked. Read More
Read MoreWho are Disqualified from Running for Public Office?
This article talks about the disqualifications for running for public office embodied in the Omnibus Election Code and Local Government Code. Read More
Read MoreWhat are the Qualifications to Run as President of the Philippines?
This article talks about the qualifications to run as President of the Philippines. The Constitution mandates that no person shall be elected President of the Philippine unless he is a natural born Filipino citizen, a registered voter, able to read and write, at least 40 years old on the day of the election and a resident of the Philippines for at least 10 years immediately preceding the election. Read More
Read MoreWhat is the Effect of the Withdrawal of Certificate of Candidacy?
This article talks about the effects of the withdrawal of a Certificate of Candidacy. Although the person ceases to be a candidate for the position, the filing of a withdrawal shall not affect the civil, criminal or administrative liabilities he may have already incurred. Read More
Read MoreWhat are the Rights of a Father Over his Illegitimate Child?
This article talks about the rights of a father over his illegitimate child. Although the mother shall have sole parental authority over the child, the father has visitation rights over his child. Read More
Read MoreExtrajudicial Settlement of Estate for Deaths from 2018 to Present
This article talks about the process of extrajudicial settlement of estate of persons who died from January 1, 2018 to present. Read More
Read MoreCan Probationary Employment Go Beyond 6 Months?
This article talks about the instances wherein the probationary employment period can go beyond 6 months, namely: 1) in apprenticeship agreements 2) employment is covered by special laws 3) practice is established by company policy or required by the nature of the work 4) to give the employee another chance to improve. Read More
Read MoreIn the Absence of a Will, When Can Brothers and Sisters Inherit from their Siblings?
This article discusses the instances when brothers and sisters can inherit from their sibling, in the absence of a will. Read More
Read MoreCan Illegitimate Children Inherit from their Parents?
This article talks about the inheritance rights of illegitimate children. Read More
Read MoreHow to Renew Your US Tourist Visa Without Going to the US Embassy
This article talks about renewing your US tourist visa without going to the US Embassy. Read More
Read MoreThe History of Penalizing Credit Card Fraud in the Philippines
This article is about punishing credit card fraud in the Philippines. Read More
Read MorePirating or Poaching Employees May be Considered as Tortious Interference
This article talks about tortious interference and its possible application to poaching and pirating of employees made by competitors. Read More
Read MoreOn Venue of Crimes: Where Do You File A Cybercrime Complaint?
This article answers the question of where to file and prosecute a criminal case involving a cybercrime. Read More
Read MoreCan You Fire an Employee for Having an Extra-Marital Affair?
This article talks about firing an employee for having an extra-marital affair based on serious misconduct, immorality and violation of company policies. Read More
Read MoreHow to Terminate an Employee Based on Loss of Trust and Confidence
This article talks about the process of terminating or firing an employee based on loss of trust and confidence. Read More
Read MoreWho Inherits When a Spouse Dies Without a Will?
This article talks about the proper heirs when a spouse dies without a will. Read More
Read MoreWho are Unworthy to be Heirs?
This article talks about the grounds for unworthiness which prevents a person from becoming an heir in both intestate and testate succession. Read More
Read MoreLearn How to Disinherit an Heir
This article talks about the process of disinheriting heirs in the testator’s last will and testament as well as the grounds or causes for disinheritance of children, descendants, parents, ascendants and spouses. Read More
Read MoreRecognition of Foreign Divorce in the Philippines
divorce, recognition of divorce, foreign divorce, divorce by foreign spouse, family law, family lawyer, family attorney, Philippine lawyer, Philippine attorney, family law firm, divorce lawyer, metro manila lawyer, metro manila attorney, best Philippine law firm, topnotch law firm, excellent Philippine lawyer Read More
Read MoreWhat is a General Information Sheet?
The General Information Sheet is a document embodying certain information about the corporation which is signed and attested to by the Corporate Secretary and usually filed annually with the SEC. Read More
Read MoreWhen are Regular Elections in the Philippines Conducted?
This article talks about the dates for regular elections in the Philippines. Elections for national, local and ARMM officials are done every three (3) years on the second Monday of May. Read More
Read MoreHow to file an Election Protest in the Philippines
This article talks about the nature of an election protest, which is concerned with the issue on who garnered the majority of votes, filed by the losing candidate within ten (10) days from the date of proclamation. Read More
Read MoreHow to Register Your Company with PEZA
This article talks about the process to have a company registered with the Philippine Export Zone Authority (PEZA). Read More
Read MoreWhat are the Requirements for PEZA Registration?
This article discusses the necessary requirements in order to register a company with the Philippine Export Zone Authority (PEZA).- Read More
You can be Punished for Being Mischievous: The Crime of Malicious Mischief
This article is about the crime of malicious mischief and its corresponding punishment. A person who shall deliberately cause damage to property of another can be guilty of the crime of malicious mischief.  Read More
Read MoreWhat are the Remedies of an Owner Against Someone Who Built Structures in his Property without his Consent?
This article talks about the rights of an owner against someone who builds structures on his land in bad faith. In cases of builders in bad faith, a property owner can choose to appropriate the structure, without any obligation to pay any indemnity except for necessary expenses, plus damages; or ask the removal of the structure at the builder’s, etc. expense, plus damages; or compel the builder to pay the value of the land, plus damages. Read More
Read MoreSummary Deportation of Foreigners in the Philippines
This article talks about the summary deportation of foreigners. This is done before the Bureau of Immigration in cases when a foreigner is overstaying, undocumented, is a fugitive from justice, has fully served the sentence of his crime which includes deportation as a penalty or a crime involving moral turpitude or the crime of failing to register with the Bureau of Immigration. Read More
Read MoreHow To Legally Move On After The Death Of Your Spouse
This article talks about the process of liquidating the conjugal properties after the death of a spouse and the consequences of failure to perform this legal requirement. If liquidation is not done, any disposition thereafter is void and if the surviving spouse remarries, a mandatory complete separation of property shall govern the subsequent marriage. Read More
Read MoreThe Unfaithful Spouse Can Lose Everything In A Legal Separation Case
This article discusses one of the effects of legal separation, which is forfeiture of the share of the guilty spouse to the net profits in the conjugal assets. Read More
Read MoreHow To Recover Possession Of Your Dog (Or Other Personal Property) Through Replevin
This article discusses the remedy of replevin under Philippine law, to recover possession of personal property unlawfully taken from another. Read More
Read MoreWhat Is A Secretary’s Certificate?
This article enumerates the general functions and duties of a Corporate Secretary. Further, this article defines a Secretary’s Certificate as a written document executed by the Corporate Secretary confirming the actions and resolutions of the Board of Directors. When regular on its face, third parties may rely on the Secretary’s Certificate without need of further investigation on the veracity of the facts contained therein. Read More
Read MoreGossiping Can Land You In Jail
This article is about the crime of gossiping or intriguing against honor and its corresponding punishment. This crime has for its principal purpose to blemish the honor or reputation of a person is punishable by imprisonment of arresto menor (1 day to 30 days) or a fine not exceeding P20,000.00. Read More
Read MoreQuestioning Corporate Takeovers Through Quo Warranto
This article discusses the legal remedy of quo warranto, as a remedy in corporate takeovers or where the eligibility or qualifications of a person who occupies the position of director, trustee, or officer of a corporation is being questioned. Read More
Read MoreCan You Work And Receive Social Security Retirement Benefit Simultaneously?
This article discusses about the grant of retirement benefit under the Social Security Act of 2018, to retirees who still wish to continue employment. Read More
Read MoreThe Crime Of Falsification Of Documents
This article is about the crime of Falsification of Documents, defines the acts amounting to falsification, the persons liable, and the penalty imposed under the Revised Penal Code of the Philippines. Read More
Read MoreWhat Is A Board Resolution?
This article defines what is a Board Resolution. Corporate powers are exercised by the Board of Directors. A Board Resolution is a formal document which serves as evidence of the actions and matters taken by the directors of the corporation in the meeting duly held. Read More
Read MoreWhat is the Crime of Perjury?
This article is about the crime of perjury and its punishment. The crime of perjury is committed by any person who shall knowingly make untruthful statements or make an affidavit, upon any material matter and required by law. It is punishable by imprisonment of up to 2 years and four months. Read More
Read MoreWHAT IS THE CRIME OF BIGAMY?
This article is about the crime of bigamy and its corresponding punishment. The crime of bigamy is committed by any person who shall contract a second or subsequent marriage before the former marriage has been legally dissolved. It is punishable by imprisonment of up to 12 years. Read More
Read MoreWhat Are The Duties Of The Corporate Secretary In Corporate Meetings?
This article is about the duties of a Corporate Secretary in corporate meetings. The Corporate Secretary is tasked to send out the notices of meetings, receive proxies, certify the existence of a quorum, note the vote of the directors, ensure the proper holding of the meeting and issue a Secretary’s Certificate to attest to what transpired during the meeting. Read More
Read MoreHow Long Is The Life Of A Corporation?
This article talks about the term of existence of a corporation. Pursuant to the Revised Corporation Code, a corporation shall have a perpetual existence, unless its Articles of Incorporation provides otherwise. The existence of a corporation may be affected by shortening the corporate term, voluntary and involuntary dissolution. Read More
Read MoreWhat Are Descriptive Trademarks?
This article discusses what are descriptive trademarks. Descriptive trademarks are those which consist exclusively of signs or of indications that may serve in trade to designate the kind, quality, quantity, intended purpose, value, geographical origin, time or production of the goods or rendering of the services, or other characteristics of the goods or services. Pursuant to Sec. 123(j) of the IP Code, such trademarks cannot be registered in the Philippines. Read More
Read MoreHow To Conduct Board Meetings In Accordance With The Revised Corporation Code
This Article discusses how to conduct board meetings under the Revised Corporation Code. Proper notices must be sent to the directors at least 2 days prior to the board meeting. The Chairman of the Board presides during the meeting and a quorum is necessary to transact corporate business. Directors who cannot attend physically may attend and vote through videoconferencing and other alternative modes of communication. Read More
Read MoreWhat Is The Crime Of Grave Threats?
The crime of grave threats is a crime committed by a person who shall threaten another with the infliction upon the person, honor or property of the latter or of his family of any wrong amounting to a crime. Read More
Read MoreWhat Are The Crimes Of Light Threats And Other Light Threats?
The crime of light threats is a crime committed by a person who shall threaten another with the infliction upon the person, honor or property of the latter or of his family of any wrong NOT constituting a crime and there is a demand for money or condition imposed. On the other hand, other light threats consist of threatening another with a weapon or other harm not constituting a crime during a quarrel. Read More
Read MoreThe Law On Christmas In The Philippines
The Philippine legal system is a civil law system. This means that the Philippines relies on laws enacted by Congress to govern all matters – including Christmas, our favorite holiday. Christmas did not just become a holiday in the Philippines by tradition or religion. As with all red letter days and public holidays, there are laws that govern the celebration of Christmas as a holiday. Read More
Read MoreComputing Employee Premium Pay In The Philippines
This article discusses how to compute night shift differential, overtime pay, and premium pay for work during weekly rest periods. Read More
Read MoreProtecting Property Rights through Adverse Claim Registration
This article discusses how to protect your interest in land or other real property by annotating an adverse claim in the certificate of title. Read More
Read MoreCurrent FDA Probiotics Regulations in the Philippines
This article discusses the Guidelines on Probiotics issued by the Food and Drug Administration of the Philippines. Read More
Read MoreJurisprudential Tests of Doing Business in the Philippines
This article discusses the various tests to determine if an entity is doing business in the Philippines. Read More
Read MoreLegal Implications of Doing Business in the Philippines
This article discusses the legal implications of doing business in the Philippines without registration or a license. Read More
Read MoreWhen Unregistered Foreign Corporations Do Business Online
This article discusses when a foreign corporation, doing business through its website or through any online platform, must register or secure a license with the Securities and Exchange Commission, and the exceptions to the requirement. Read More
Read More2021 Philippine Holidays
This article discusses the Philippine Holidays for the year 2021, as declared under Proclamation No. 986. Read More
Read MoreNew FDA Requirement on Licensing Household Hazardous Substances
This Article discusses the new FDA Circular 2020-025 or the Implementing Guidelines for Administrative Order No. 2019-019 which now requires registration of household hazardous goods in the Philippines. The requirement of registration was previously removed by the Philippine Food and Drug Administration, but reinstated and to take effect beginning 03 September 2020. Read More
Read MoreIs Hazing Prohibited in the Philippines?
Hazing in the Philippines is prohibited under Republic Act No. 8049 as amended by Republic Act No. 11053. Read More
Read MoreExtension of Floating Status or Suspension of Employment During a Pandemic
DO 215 allowed the extension of suspension of employment for a period not exceeding six (6) months. Read More
Read MoreEverything You Need to Know About Placing an Employee on Floating Status
This Article discusses the nature, effects, procedure and requirements, and legality of placing employees under floating status or temporary lay-off. Read More
Read MoreHow to Form a Corporation in the Philippines
In order to set up a corporation in the Philippines, the incorporation process is lodged with the Securities & Exchange Commission (SEC). Read More
Read MoreIf the Wife Gives Birth to a Child of Another Man, what is the Status of the Child?
If the Wife Gives Birth to a Child of Another Man, the child is considered as legitimate unless the husband impugns the legitimacy of the child within the period prescribed by law. Read More
Read MoreIs the Act of Spreading Nude Photos or Sex Videos in the Internet Punishable in the Philippines?
Spreading the nude photos or sex videos of another without his or her consent is punishable under Republic Act No. 9995, otherwise known as the Anti-Photo and Video Voyeurism Act of 2009. Read More
Read MoreWhen Commenting in Social Media becomes Cyber Libel
This article discusses whether commenting in social media can give rise to liability for cyber libel. Read More
Read MoreIs there Liability for Cyber Libel for Sharing, Commenting Retweeting, or Liking on Social Media?
This article answers the question- will someone who posts, likes, or retweets an openly libelous post be liable for the crime of cyber libel. Read More
Read MoreWhen is a Person Liable for the Crime of Cyber Libel?
This article focuses in-depth on the law, elements, penalty imposed, and legal treatment of the crime of cyber libel in the Philippines. Read More
Read MoreWho has the Right to Decide on the Funeral Arrangements of the Deceased?
The following hierarchy shall be observed in giving preference to decide on the funeral arrangements of the deceased: the spouse, descendants in the nearest degree, ascendants in the nearest degree and brothers and sisters Read More
Read MoreHow to File a Criminal Case for Adultery Against your Wife in the Philippines
You can file a criminal case for adultery against your wife and her paramour with the Office of the City Prosecutor in the city where they had sexual relations. You need to undergo the process of preliminary investigation. Read More
Read MoreWhat are the Rights of Work from Home (WFH) Employees?
Work from Home (WFH) employees are entitled to rights granted by the Labor Code and the Telecommuting Act of 2018. Republic Act No. 11165, otherwise known as the Telecommuting Act, recognizes telecommuting, or work from an alternative workplace with the use of telecommunications and/or computer technologies. A work from home arrangement can be properly considered as telecommuting. Under this law, WFH employees must be given fair treatment. Of course, rights come with responsibilities. Hence, the WFH employee must still abide by the policies of the company. The employer and the employee must adhere to and comply with the Telecommuting Program of the company which stipulates provisions on eligibility, code of conduct, use of equipment, work hours, data privacy, etc. Read More
Read MoreWho Can File a Case for Adultery?
Only the offended husband can file a criminal case for adultery against his wife who had sexual intercourse with another man. Article 333 of the Revised Penal Code penalizes a married woman for having sexual intercourse with another man who is not her husband. That other man or paramour is also held liable if he knew the woman to be married and still had sexual intercourse with her Read More
Read MoreWhat is the Dominancy Test in Assessing Trademarks?
The dominancy test focuses on the similarity of the prevalent or dominant features of the competing trademarks that might cause confusion, mistake, and deception in the mind of the purchasing public. Read More
Read MoreWhat is the Holistic Test in Assessing Trademarks?
The Holistic Test requires that the entirety of the marks in question be considered in resolving confusing similarity of competing trademarks. Read More
Read MoreIs an Employer Mandated to Provide Work From Home (WFH) Arrangements?
This article discusses the Telecommuting Act, or Republic Act No. 11165, and whether it is mandatory for employers to provide work from home arrangements. Read More
Read MoreWhen do You Get your Final Pay When You Resign?
This article discusses when you get your final pay when you resign. It discusses recent labor department issuances. Ultimately, you get your final pay within 30 days from resignation. Read More
Read MoreA 5-Minute Simple Guide to Starting a Business in the Philippines
This Article discusses the registration requirements for starting or opening a business in the Philippines. Read More
Read MoreCOVID-19 in the Workplace: A Quick Employer FAQ (Updated as of 20 August 2020)
This article is a brief employer's guide to dealing with COVID-19 in the workplace. It discusses about the employer's obligations regarding contact tracing, reporting, office protocol, COVID-19 testing of employees, and medical costs and expenses relating to COVID-19. This article also mentiones the DOH and the local government units as the principal agencies responsible for contact tracing. Read More
Read MoreHow to Compute Your Retirement Pay
This article answers basic questions on how to compute employee retirement pay under Philippine Law. This helps employers compute the legal retirement pay due. Read More
Read MoreWhat to Do If your Spouse Has Been Missing for Many Years and You Wish to Remarry
This Article tackles the requirements, procedure and effects of a declaration of presumptive death on spouse who goes missing and the effects of reappearance. Read More
Read MoreCan a Muslim-Convert Marry Again?
This article discusses the legal issue of bigamy after a subsequent marriage celebrated under Muslim laws. Specifically, it answers the question: Can a Muslim-Convert who is already married marry again during the existence of the first non-Muslim marriage, without being liable for bigamy? Can a married person convert to another religion for the purpose of marrying another? A spouse, by simple expediency of converting to the Muslim faith, cannot validly marry another, without having the first marriage legally dissolved. This is the answer to the question addressed by this article. Read More
Read MoreHow Much Support Should a Parent Give his Child?
This article discusses the legal basis and parameters of child support. How much child support must a parent actually give a child? Up to when should support be given? Whose obligation is it to give support to a child? These are some of the questions answered in this article. Read More
Read MoreHow to Claim SSS Unemployment Benefits
This article is a brief FAQ on how to apply for SSS unemployment benefits in the Philippines. It talks about the applicable law, those eligible to apply, those disqualified from applying, the documents and requirements for the application, the process, and the limitations of the law in claiming SSS unemployment benefits. Read More
Read MoreNotarization through Videoconferencing During the Quarantine
In response to the COVID-19 pandemic, the Philippine Supreme Court issued A.M. No. 20-07-04-SC known as the 2020 Rules on Remote Notarization of Paper Documents. This issuance allows the notarization of documents in the Philippines through videoconferencing, where the notary public or at least one of the parties to the document is in a locality under community quarantine due to COVID-19. Read More
Read MoreFiling a Collection Suit for Sum of Money
Do you want to collect from the people who borrowed money from you? Where payment of such money claim is the principal relief sought, filing a collection suit is proper. Read More
Read MoreQ & A on Small Claims Cases in the Philippines
Do you wish to collect money from someone who owes you but cannot afford to spend on heavy legal fees? Is your lessee not paying his rent but you do not want the hassle of a long court trial? Did you deliver products to your customer yet he did not pay you? Have you experienced […] Read More
Read MoreSecuring a License to Operate with the Food and Drug Administration in the Philippines: What is a License to Operate and When is it Needed?
What is a License to Operate (“LTO”)? A License to Operate or LTO is a license which must be secured in order to operate or establish an establishment prior to engaging in the manufacture, importation, exportation, sale, offer for sale, distribution, transfer, and where applicable the use, testing, promotion, advertisement, and/or sponsorship of food, drugs, […] Read More
Read MoreE-Portal: Filing and Application for a License to Operate (LTO) as a Cosmetic / Drug /Medical Device / Food Manufacturer, Importer Or Distributor with the Food and Drug Administration of the Philippines
The LTO Application Process Is Now Streamlined On 02 May 2016, the Food and Drug Administration (“FDA”) adopted a new application process and form for a License to operate (“LTO”). The application process is now navigable and accessible through the electronic portal found at the FDA website (www.fda.gov.ph). The electronic process for LTO application has […] Read More
Read MoreTaxes and Transfer of Stocks Listed and Traded through the Local Stock Exchange
New Tax Rates on Stock Transfers This article discusses the tax involved in the transfer of stocks which are listed and traded through the Local Stock Exchange. In stock transfers, the tax to be paid is called a Percentage Tax, which is a business tax imposed on stock transfer transactions such as the sale, barter, […] Read More
Read MoreProhibited Claims in Food Product Labels under FDA Regulations and Philippine law
Purpose of the Law To protect the public against dishonest or misleading advertising or promotion, and to ensure that every consumer acquires sufficient knowledge to be an informed consumer, particularly in the choice of the consumer products that he or she wishes to purchase or consume, the Food and Drug Administration (“FDA”) issued Administrative Order […] Read More
Read MoreWhat are the FDA Mandatory Label Information for Food Products?
Mandate of the Food and Drug Administration Republic Act No. 3720, or otherwise known as the Food, Drug, and Cosmetic Act, is the law creating the Food and Drug Administration (“FDA”), which is under the Office of the Secretary of the Department of Health. The FDA is tasked by law to prescribe standards, guidelines, and […] Read More
Read MoreFDA Requirements for the Issuance of an Authorization for the Sale, Manufacture, Importation or Distribution of a Medical Device in the Philippines
What is a medical device? The regulations issued by the Food and Drug Administration (FDA) of the Philippines defined a ‘medical device’ as any instrument, apparatus, implement, machine, appliance, implant, in-vitro reagent or calibrator, software, material, or other similar or related article intended by the manufacturer to be used alone, or in combination, for human […] Read More
Read MoreAn FDA Requirement: How to Maintain a Product Information File?
FDA Issuance Requiring a Product Information File With the end in view of ensuring safety, quality and/or efficacy of the cosmetic products that companies are placing in the market, the Food and Drug Administration (FDA) issued FDA Circular No. 2018-001, which requires a Marketing Authorization Holder (MAH) to keep and maintain a Product Information File. […] Read More
Read MoreNew Procedure: How to Liquidate a Closed Bank in the Philippines
The Supreme Court enacts New Rules of Procedure on Liquidation of Closed Banks The Supreme Court recently enacted new rules of procedure for the liquidation of closed banks in the Philippines. Scheduled to take effect on April 16, 2020, A.M. No.19-12-02-SC governs the Rules on Liquidation of Closed Banks (Rules). The Rules apply to local […] Read More
Read MoreHow to File an Admiralty or Maritime Claim in the Philippines under the New Rules of Procedure
New Rules of Procedure for Admiralty or Maritime Claims in the Philippines With the end in view of providing a fast, reliable, and efficient means of recourse to Philippine courts and enhancing the administration of justice in Admiralty cases in the Philippines, the Supreme Court promulgated Administrative Matter No. 19-08-14-SC, or otherwise known as the […] Read More
Read MoreOn Real Estate Investment Trust (REIT): How to Engage in a REIT Business in the Philippines
Do you want to participate in the real estate business in the Philippines? Do you know where to invest your money in real estate and create a real estate investment trust? If you don’t have the necessary knowledge about real estate investment, this article will help you know the basics about real estate investment trust. […] Read More
Read MoreHow to Apply for a Cosmetic Product Notification in the Philippines
Regulatory Issuance Governing Cosmetic Product Notification From manual application process, the application for cosmetic product notification in the Philippines can now be done online. Aiming to streamline the process by updating the submission of application requirements from the previously manual form to online submissions, the Food and Drug Administration (FDA) allowed online application process. This […] Read More
Read MoreGuidelines of the Food and Drug Administration in the Commercial Display, Selling, Promotion, and Advertising of Alcoholic Beverages, and other Beverages that Contain Alcohol
Philippine Regulatory Agency for Beverages with Alcohol In the Philippines, the proper government agency that is authorized under the law to act as the policy formulation and sector monitoring of the Secretary of the Department of Health, on matters pertaining to food, drugs, traditional medicines, cosmetics and household products containing hazardous substances, is the Food […] Read More
Read MoreFood Product Labeling Requirements of the FDA
Regulations that Govern Food Product Labelling in the Philippines The Philippines’ Food and Drug Administration (FDA) issued Administrative Order No. 2014-0030, or otherwise known as the Revised Rules and Regulations Governing the Labeling of Prepackaged Food Products (FDA Labeling Regulations). It covers the labeling of all prepackaged food products, including food supplements, whether locally manufactured, […] Read More
Read MoreCosmetic Product Labeling Regulations of the Food and Drug Administration
A cosmetic product is one of the health products regulated by the Food and Drug Administration (FDA) of the Philippines. The office of the FDA responsible in regulating cosmetic products and ensuring compliance with the regulatory approvals imposed by the FDA is the Center for Cosmetics Regulation and Research of the FDA. What is a Cosmetic […] Read More
Read MoreDrug Labeling Requirements of the Food and Drug Administration
Governing Regulation for Drug Labelling Requirements The primary sources of information for consumers are the labels and labeling materials, as they provide useful information such as those dealing with the safe and effective use of a drug product (e.g. indication(s), pharmacologic class and dosage), and information dealing with quality (e.g. manufacturing and expiration dates, registration […] Read More
Read MoreApplying for a Certificate of Product Registration for Food Supplements with the Food and Drug Administration (FDA) of the Philippines
What is a food supplement? A food or dietary supplement is a processed food product intended to supplement the diet that bears or contains one or more of the following dietary ingredients: vitamin, mineral, herb, or other botanical, amino acid, and dietary substance to increase the total daily intake in amounts conforming to the latest […] Read More
Read MoreEffect, Prohibition, and Penalties for Adulteration and Misbranding of Drug, Food, Cosmetic Products and Medical Devices in the Philippines
Prohibition on Adulteration or Misbranding The adulteration or misbranding of any health product and the manufacture, importation, exportation, sale, offering for sale, distribution, transfer, non-consumer use, promotion, advertising, or sponsorship of any health product that is adulterated, unregistered or misbranded. Under the law, health products include food, drugs, cosmetics, devices, biologicals, vaccines, in-vitro diagnostic reagents […] Read More
Read MoreHow to File a Petition for Writ of Habeas Data
Is your right to privacy in life, liberty or security is violated or threatened? If so, you may file a petition for the issuance of a writ of habeas data. A. M. No. 08-1-16-SC provides for the rules on the writ of habeas data. What is a writ of habeas data? The writ of habeas […] Read More
Read MoreClarifying Mandatory Requirements for FDA Registration in the Philippines
Do you need to employ a pharmacist, even if your company is selling cosmetic products or medical devices, as a requirement for registration with the Food and Drug Administration (FDA) of the Philippines, or in applying for an LTO with the FDA? To answer this question, we need to understand the legal requirements governing registration […] Read More
Read MoreHow to Apply for Variations with the Food and Drug Administration
Do you want to add production lines or change your establishment’s business name? Do you want to transfer the location of your manufacturing plant or add additional floor in your building of production? Do you want to add additional activity of your business such as online ordering and delivery? You can do these provided you […] Read More
Read MoreFiling a Damage Suit in the Philippines
What are ‘damages’ under Philippine law? ‘Damages’ is a term defined by the Supreme Court in the case of MEA Builders, Inc. vs. Court of Appeals, G.R. No. 121484, 31 January 2005, as the sum of money which the law awards or imposes as a pecuniary compensation, a recompense, or satisfaction for an injury done […] Read More
Read MoreHow to Legally Adopt a Child in the Philippines
If you want to legally adopt a child in the Philippines, this guide is for you. We outline in this article the procedure and requirements to adopt a child in the Philippines. Read More
Read MoreWhat is Calamity Leave?
On 08 February 2012, the Civil Service Commission passed Resolution No. 1200289, providing a five-day special emergency leave to government employees directly affected by natural calamity/disaster. The Resolution did not define calamity or disaster but under Republic Act No. 10121, or otherwise known as the Philippine Disaster Risk Reduction and Management Act of 2010, State […] Read More
Read MoreHow to be a Legal Guardian of a Minor?
We discuss in this article the procedure to be appointed as Legal Guardian of a Minor. In the Philippines, the law provides that the father and the mother shall jointly exercise legal guardianship over the person and property of their minor child wihtout court appointment. If the parents are not available, the court-appointed guardian acts in their stead, in all matters where the decision of a parent is required. Read More
Read MoreA Quick Remedy to Obtain Child Custody: File a Petition for Habeas Corpus
This article provides an expeditious remedy to obtain custody over a minor child, through a Petition for Habeas Corpus, its legal basis, procedure and effects.- Read More
How to Claim Custody Over a Minor Child
This article provides the solution to the question "How do you Claim Custody Over a Minor Child?". It discusses the grounds, procedure and applicable law.  Read More
Read MoreHow to End your Marriage thru Legal Action in the Philippines
How do you end a marriage in the Philippines? This article discusses about the procedure and nuances of filing a Petition for Declaration of Absolute Nullity of Marriage. Read More
Read MoreHow to File for Annulment in the Philippines
This is an article about filing for annulment of marriage in the Philippines. It discusses the procedure, grounds, form, and effects of filing a petiton for annulment. Read More
Read MoreFrequently Asked Questions (FAQs) in Legal Separation in the Philippines
This article discusses how to be legally separated from your spouse, the nature, process, grounds, and effects of filing a Petition for Legal Separation in the Philippines. Read More
Read MoreWhat is Syndicated Estafa?
This article speaks about the crime of syndicated estafa punished under PD 1689. It is the highest form of estafa, and penalized with the highest penalty, which is life imprisonment. Read More
Read MoreNew Penalties for Estafa or Swindling
This article discusses Estafa or criminal fraud under Republic Act No. 10951, which adjusted the legal basis for the penalties imposed under Philippine law. Read More
Read MoreAnother Type of Fraud: Estafa by means of Deceit
This article outlines a form of criminal fraud in the Philippines called Estafa through deceit or false pretenses, the nature of the crime, legal bases or elements, and ways of commiting this kind of estafa.- Read More
New Normal: Electronic Filing of Complaints and Posting of Bail
This article discusses a "new normal" in the Philippines, which is the electronic filing of criminal complaints and Information and electronic posting of bail.  Read More
Read MoreAnother type of Fraud: Simple Estafa by Misappropriation
This article discusses Estafa or criminal fraud, through conversion or misappropriation, under Article 315(1)(b) of the Revised Penal Code of the Philippines. Read More
Read MoreWhat Happens During a Preliminary Investigation?
This article discusses the nature and scope of a preliminary investigation in the Philippines, and procedure, requirements, and the procedure for filing a case in the Philippines. Read More
Read MoreThe Financial Rehabilitation and Insolvency Act of 2010
This article is about the Philippines' Financial Rehabilitation and Insolvency Act of 2010, its nature, scope and coverage for businesses, process of application and other legal requirements. Read More
Read MoreThe Threefold Duties of a Director of a Corporation
This article discusses the duties, functions and responsibilities of a director of a corporation, and the board of directors, towards the corporation, the stockholders and under Philippine law. Read More
Read MoreThe Corporation as a Business Structure
This article discusses the corporation as a business structure, its nature, formation, function, ownership of shares and other matters dealing with a corporation as a business entity in the Philippines. Read More
Read MoreOn Corporate Combinations: Acquisitions, Merger and Consolidation of Corporations
This article discusses about the nature, process, nuances, and consequences of Corporate Combinations, Acquisitions, Merger and Consolidation of Corporations in the Philippines. Read More
Read MoreIntra-corporate Disputes and Arbitration Under the Revised Corporation Code
This article discusses the nature, definition, and the different types of intra-corporate disputes and controversies under the Revised Corporation Code of the Philippines Read More
Read MoreOne Person Corporation: A New Form of Corporation
This article discusses setting up, requirements, and other ins and outs of a One Person Corporation (OPC) in the Philippines. Read More
Read MoreOn Corporate Officers: Who Can Be Officers of a Corporation?
This article deals with the different types, functions, qualifications and disqualifications of coprorate officers under the Revised Corporation Code of the Philippines. Read More
Read MoreCrimes punished under the New Corporation Code
This article discusses the penal provisions or acts punished, and penalties imposed under Republic Act No. 11232 or Revised Corporation Code of the Philippines. Read More
Read MoreFrequently Asked Questions (FAQs) on Insurance
This articles discusses common or frequently asked questions on contracts of insurance, and insurance law in the Philippines. Read More
Read MoreFrequently Asked Questions (FAQs) on the General Community Quarantine (GCQ)?
This articles features frequently asked questions (FAQss) on the General Community Quarantine in the Philippines Read More
Read MoreA Transition from Enhanced Community Quarantine to Modified Enhanced Community Quarantine (ECQ to MECQ)
This article discusses the change from Enhanced Community Quarantine to Modified Enhanced Community Quarantine, the differences between them, and the restrictions, conditions, and regulations that will be in place during the period. Read More
Read More(UPDATED) A 5 Minute Business Guide to Surviving the COVID-19 Pandemic
This is a buisness guide to surviving the COVID-19 Pandemic. It outlines the Philippine government measures reliefs, programs, and remedies that business owners can employ. Read More
Read MoreMarital Rape: Can a Husband Be Liable for Rape Committed Against His Wife?
Spousal Procreation: A Right or Responsibility? Article 68 of Executive Order No. 209, or otherwise known as the Family Code of the Philippines obligates the spouses to love one another, viz: “Art. 68. The husband and wife are obliged to live together, observe mutual love, respect and fidelity, and render mutual help and support.” The […] Read More
Read MoreDamages Awarded under Philippine Law
This article discusses the nature and kinds of damages awarded by courts in the Philippines, including the legal bases for their grant. Read More
Read MoreThe Basics of an Insurance Contract
This article disucsses the nature and basic implications of an insurance contract in the Philippines, including the applicable law and Supreme Court cases dealing with the subject. Read More
Read MoreWho Has Parental Authority Over an Illegitimate Child?
This article discusses which parent, the father or mother, shall have legal custody over an illegitimate child. Read More
Read MoreHomicide or Murder? What makes the Difference in Philippine Laws?
This article explains the difference between the crimes of murder and homicide, their respective elements and penalties. Read More
Read MoreOn Bona Fide Occupational Qualification: Can You Be Dismissed Based on Your Weight?
This article discusses if weight is a valid occupational requirement, and whether companies can impose certain qualifications employment requirements. Read More
Read MoreHow to Make a Last Will and Testament in the Philippines
This article discusses how to prepare and draft a Last Will and Testament in the Philippines, particularly a Notarial Will, and how to comply with the legal requirements for the validity of a Last Will and Testament in the Philippines. It also discusses relevant Supreme Court cases on last wills and testaments in the Philippines. Read More
Read MoreSuing for Unfair Competition in the Philippines
This article discusses about the crime of unfair competition in the Philippines, the elements of the crime, the penalty for its commission, and the acts that must be proven to be convicted of the offense. It also discusses relevant Supreme Court cases on unfair competition in the Philippines. Read More
Read MoreCan You File Estafa and BP 22 at the Same Time?
This article discusses the crimes of Estafa and BP 22 or the Bouncing Checks Law in the Philippines, the respective elements of the crimes of Estafa and BP 22, and whether these two (2) crimes can be filed simultaneously. It also discusses relevant Supreme Court cases on Estafa and BP 22 in the Philippines. Read More
Read MoreOn Forgery of Checks : Who Bears the Loss?
This article discuses the issue of forgery of checks and other negotiable instruments like bank drafts and other demand or bearer notes, and answers the question of their validity, and who is liable if they are forged. It also discusses relevant Supreme Court cases on checks and forged negotiable instruments in the Philippines. Read More
Read MoreIncurred Damage or Loss in Land Registration? Go after the Assurance Fund
This article discusses on what can be done if a person sustains damage, injury or is deprived of ownership of land as a consequence of fraudulent registration, or any error, omission or mistake in registration, and the remedies that a person can resort to. It also discusses relevant Supreme Court cases on claims that can be made against the Assurance Fund. Read More
Read MoreChange of Name and Sex on the Ground of Sex Change
This article discusses whether a person can have his or her birth certificate corrected, or entries in the Civil Registry changes, after a sex change or sex reassignment through surgery. It also discusses relevant Supreme Court cases on change of name and sex on the ground of sex change or sex reassignment surgery. Read More
Read MorePenalties for Unlawful Acts Under the New Firearms Law of the Philippines
This article discusses Republic Act No. 10591, or the Comprehensive Firearms and Ammunition Regulation Act, the new crimes and penalties punished under the law, and also the new crime of Use of an Imitation Firearm, as punished under RA 10591. Read More
Read MoreRA 10883: The New Anti-Carnapping Law of the Philippines
This article discusses Republic Act No. 10883, or otherwise known as the New Anti-Carnapping Act of 2016 (RA 10883), a law which punishes carnapping in the Philippines, its penalties and the elements of the crime. Read More
Read MoreComputer-related Identity Theft is a Serious Cyber Crime
This article discusses the cyber crime called Computer-related Identity Theft in the Philippines, its legal basis and the penalties for the cyber crime. Read More
Read MoreCan an Employee be Dismissed due to Pregnancy Out of Wedlock?
This article answers the question "can an employee be dismissed due to pregnancy out of wedlock?" by providing three (3) cases decided by the Philippine Supreme Court. Read More
Read MorePunishable Acts Under Republic Act No. 11332
This article is about the acts punishable under Republic Act No. 11332 (RA 11332) or the Mandatory Reporting of Notifiable Diseases and Health Events of Public Health Concern Act Read More
Read MoreGrounds for Declaration of Nullity, Annulment, and Legal Separation
This is an article about the grounds and effects of a declaration of nullity of marriage, annulment of marriage, and legal separation. Read More
Read MoreBail in the Philippines: Matter of Right or Discretion?
This article discusses an individual's right to bail in the Philippines, bail as a matter or right and bail as a matter of discretion, and the constitutional rights and limitation of the right to bail. Read More
Read MoreWhen can a Corporation be Liable for Tort or Damages?
This article deals with corporate liability for tort, and addresses the question on whether a corporation can be held liable, as a juridical entity, for damages arising from negligence. Read More
Read MoreWhen Can One Invoke the Right to Privacy in Facebook Posts?
This article discusses the right to privacy, in relation to posting on social media, particularly on Facebook. Read More
Read MoreOn Death and Taxes: Estate Tax under the TRAIN Law
This article discusses about computing and paying Estate Tax under the TRAIN Law in the Philippines. This applies to succession, settlement of estate, inheritance, estate planning, since Estate tax is a liability to be paid in relation to these aspects of Philippine law on succession. Read More
Read MoreWhen are Checkpoints Valid and What do you do?
This article discusses the legality of government checkpoints, and your rights during searches done in airports, seaports, moving vehicles, ships, and airplanes. Read More
Read MoreWhat Are the Offenses Punishable Under Republic Act No. 11469 or the Bayanihan to Heal as One Act?
This article discusses the penal provisions, crimes and penalties punished or imposed under Republic Act No. 11469 or the Bayanihan to Heal as One Act. Read More
Read MoreLibel in the Cyberspace
Protecting Cyberspace from Cybercrimes In this modern age, with the emergence of mobile internet and wireless broadband subscriptions, almost all our conversations take place in the cyberspace. As of 2020, there are 1.69 billion Facebook users in the world and 330 million monthly active users and 145 million daily active users on Twitter. [1] We often […] Read More
Read MorePrice Manipulations in the COVID Situation: Punishing Hoarding, Profiteering, and Cartel during crises in the Philippines
This article discusses about consumer protection from hoarding, profiteering, and cartels, during the COVID-19 crises under the Price Act and Bayanihan to Heal as One Act. Read More
Read MoreLegal Employee Retrenchment due to COVID-19
This is how legal retrenchment of employees can be implemented due to COVID-19 in the Philippines. Read More
Read MoreWhat is a Trademark?
This article discusses about use, function and ownership of trademarks under Philippine law. Read More
Read MoreWhat Do You Get When You Resign?
This Article applies to voluntary employee resignation. It discusses what an employee who resigns will actually receive from the company upon resignation. Read More
Read MoreHow to Properly Adopt Remedial Measures in the Company to Mitigate Losses due to COVID-19
The Philippine Government recently issued Guidelines in response to the Ongoing Outbreak of the Corona Virus Disease 2019 or COVID-19 Read More
Read MoreA Guide to Dealing with Suspension of Work Due to Calamities
This article discusses the Philippine government's policy or DOLE's regulations on suspension of work due to natural calamities and disasters. Read More
Read MoreWhy Should You Register Your Trademark?
This article discusses the many benefits of trademark registration, including, protection of business identity, access to legal remedies, and prevention of trademark infringement. Read More
Read MoreHow to Sue Someone before the IPO for Copying your Trademark
This is an article on how to sue someone before the Intellectual Property Office for copying your trademark or trademark infringement. Read More
Read MoreHow to Sue Your Husband for Marital Infidelity Committed Abroad
This article tells you how to sue your husband for marital infidelity committed abroad. Read More
Read MoreHow to Maintain your Trademark in the Philippines in 2 Easy Steps
This article is a quick guide to maintaining your trademark registration in the Philippines. Read More
Read MoreHow to Renew a Trademark Registration in the Philippines in 3 Easy Steps
This article outlines the short steps to renew a trademark registration in the Philippines. Read More
Read MoreHow to Register a Trademark in the Philippines in 3 Easy Steps
We can help you register your trademark in three (3) easy steps. This article outlines the steps for trademark registration in the Philippines. Read More
Read MoreFrequently Asked Questions (FAQs) on Philippine Trademark
This is an FAQ guide on Trademark Registration in the Philippines. Read More
Read MoreHow to (Legally) Move On After Being Abandoned by Your Spouse
To be abandoned by a spouse is indeed a torment which you would not even wish on your enemy. How does one move on from abandonment? In this article, we discuss the effects of abandonment on one spouse and how he or she can move on under the Family Code of the Philippines and relevant laws. Read More
Read MoreHow To Sue Your Wife For Adultery In The Philippines
The sound of wedding bells and the tune of “Here Comes the Bride” are wonderful sounds which most people aspire to hear. However, once the ceremonies are over and the fairy tale ends, there are some grim realities that beset unfortunate married couples. One of them is the infidelity committed by one of the parties. […] Read More
Read More2020 Annual Report Requirement for Foreign Nationals
As it is the start of the year, all foreign nationals are reminded of complying with the Bureau of Immigration’s Annual Report Requirement for 2020 (http://immigration.gov.ph/41-annual-report-a-r). Foreign nationals holding Temporary Visitor’s Visas or Tourist Visas are excluded from this requirement. Mandatory under Philippine Law The annual report is mandatory under Republic Act No. 562 otherwise […] Read More
Read MoreAnalyzing Whether a Business Conduct Contravenes Competition Law Using the Structure-Conduct-Performance Framework
What is Structure-Conduct-Performance Framework To determine whether a business conduct contravenes antitrust laws, it is necessary to make a competition analysis. The analytical framework that is commonly used in competition analysis is the Structure-Conduct-Performance Framework (SCP). The SCP approach assumes that there is a stable, causal relationship between the structure of an industry, firm conduct […] Read More
Read MoreDawn Raids? No problem. The Rule on Administrative Search and Inspection under the Philippine Competition Act
The Philippine Competition Act (“PCA”) prohibits certain anticompetitive conduct. The Philippine Competition Commission (“PCC”) is tasked with the enforcement of the law. To successfully prosecute violators of the PCA, the PCC, under the the Philippine Supreme Court issued A.M. 19-08-06-SC, is empowered to conduct dawn raids, and apply for an inspection order before the Special Commercial Court of the place to be inspected is located. Read More
Read MoreWho let the Dogs Out? What to do When your Dog Bites a Person
Under Republic Act No. 9482, otherwise known as the Anti-Rabies Act of 2007, the pet owner has responsibilities when his pet dog bites another. He must assist the Dog bite victim immediately and shoulder the medical expenses incurred and other incidental expenses relative to the victim’s injuries Read More
Read MoreA Law that Can Protect Bloggers?
Approved by President Rodrigo R. Duterte on August 30, 2019, Republic Act No. 11458 (RA 11458) was passed, expanding the existing protection given to print and traditional media publishers, editors or reporters, and granting the same protection to “duly recognized or accredited journalist, writer, reporter, contributor, opinion writer, editor, columnist, manager, media practitioner involved in the writing, editing, production, and dissemination of news for mass circulation, of any print, broadcast, wire service organization, or electronic mass media, including cable TV and its variants.” Read More
Read MoreCommunity Service as A Penalty for a Felony
Under the Community Service Act, the court may, in its discretion, and lieu of service in jail, require that the penalties of arresto menor and arresto mayor be served by the defendant by rendering community service in the place where the crime was committed, and under such terms as the court shall determine, taking into consideration the gravity of the offence and the circumstances of the case. Read More
Read More2020 Philippine Holidays
On November 15, 2019, President Duterte passed Proclamation No. 845, which designates the Philippine holidays, specifically regular and special non-working days, for the year 2020. Read More
Read MoreA Guide on One Person Corporations in the Philippines
A One Person Corporation (”OPC”) may be formed in the Philippines whereby the OPC is composed of a single stockholder who can only be a natural person, trust or estate. Its term of existence is perpetual but in case of a trust or estate, the term shall be co-terminous with the existence of the trust or estate. Read More
Read MoreA Law for Left Handed-Students
Approved by the President on August 22, 2019, Republic Act No. 11394 (RA 11394) makes its obligatory for all schools, both public and private, that makes use of armchairs in the classroom, to provide neutral desks to all students. RA 11394 even defined a neutral desk to mean “a table or an armchair that is suitable for both right-handed and left-handed students.” The law further required that at least ten percent (10%) of the student population be provided with neutral desks. Read More
Read MoreAn Unregulated Franchising Environment (updated October 2019)
In the Philippines, there is no requirement to register a franchising agreement. A Technology Transfer Arrangement need not be registered with the Intellectual Property Office if it complies with the mandatory provisions and does not contain any of the prohibited clauses. On the other hand, some countries require that the franchise agreement be registered and oftentimes updated annually. Parenthetically, since there is no law specifically addressed at franchising, there is also no government agency charged with regulating the business of franchising in the Philippines. Perhaps, it is about time that Congress enact a law on franchising to protect the legitimate players in such thriving industry. Read More
Read MoreTemping in the Judiciary? Judges-at-Large Law Passed
Prompted by the heavily clogged dockets of the courts, the lack of magistrates and the seemingly inadequate number of courts to hear and decide the mounting scores of cases remain in the Philippine Judicial System, the Congress passed Republic Act No. 11459 otherwise known as the Judges-at-Large Act of 2019. Read More
Read MoreOn Video and Audio Recording using your Mobile Phone
With advancements in mobile technology, the average person can now record a documentary, or even an explosive exposé, merely using one’s mobile phone, with crystal clear picture and sound, that may even impress professionals. However, you must always be careful with who and what you record with your phone. Chances are, if you recorded a […] Read More
Read MoreCyber Libel: Liability for Posting in Social Media
In response to developments in technology and the ever-growing use of multiple forms of social media in the Philippines, Congress enacted Republic Act No. 10175, also known as the Cybercrime Prevention Act of 2012. Law Defining Cyber Libel R.A. 10175 deals with many crimes, including byber libel. Sec. 4(c)(4) of R.A. 10175 states: “(4) Libel. […] Read More
Read MoreAntitrust/ Competition Law: Investigation of Administrative Cases involving Anti-Competitive Conduct before the Philippine Competition Commission
Antitrust/ Competition Law: Investigation of Administrative Cases involving Anti-Competitive Conduct before the Philippine Competition Commission* The Philippine Competition Commission (“PCC”) shall have original and primary jurisdiction over the enforcement and implementation of Republic Act No. 10667, otherwise known as the Philippine Competition Act (“PCA”).[i] It has the power to conduct inquiries and investigate, upon complaint […] Read More
Read MoreAntitrust/ Competition Law: Relevant Product Market and the Hypothetical Monopolist Test
Antitrust/ Competition Law: Relevant Product Market and the Hypothetical Monopolist Test* Market definition is usually the first step in competition analysis because the concepts that will be used in the analysis occur in the market (i.e. market power, competition, statutory restrictions, etc.). Furthermore, defining the market is relevant in calculating and determining variables such […] Read More
Read MoreAntitrust/ Competition Law: Vertical Restraints and Exclusive Dealing
Antitrust/ Competition Law: Vertical Restraints and Exclusive Dealing* A vertical agreement is an agreement or concerted practice entered into between two or more undertakings each of which operates, for the purposes of the agreement or the concerted practice, at a different level of the production or distribution chain, and relating to the conditions under which […] Read More
Read MoreAntitrust/ Competition Law: Abuse of Dominant Position in Light of Tying Arrangements or Tie-in Sales
Antitrust/ Competition Law: Abuse of Dominant Position in Light of Tying Arrangements or Tie-in Sales* Have you ever experienced a situation wherein your purchase of one product requires you to buy another product? This practice may be classified as a tying arrangement or a tie-in sale which may be considered a violation of Republic Act […] Read More
Read MoreEmployer’s SSS Condonation Program ends September 2019
The Social Security System (SSS) recently issued a reminder/notice to all employers of the current SSS Condonation Program and the fast approaching deadline, to avail of the program. Under the law, a penalty of three percent (3%) per month is assessed on unremitted contributions. This penalty continues to accrue and to compound, from the date […] Read More
Read MoreNo more fees for First-Time Job Seekers
The Philippine government has recently passed Republic Act 11261, also known as the First Time Jobseekers Assistance Act. Passed primarily for the benefit of a first time jobseeker, the hopeful applicant, who is applying for his or her first job, will be exempted from payment of fees or charges which may be assessed by local […] Read More
Read MoreEnergy Conservation given the Green Light under Republic Act No. 11285
Green energy advocates and conservationists, rejoice! Republic Act No. 11285 (RA 11285), otherwise known as the Energy Efficiency and Conservation Act, is now binding law in the Philippines. With RA 11285, energy conservation and efficiency are no longer terms within the realm of policy statements. Energy conservation and efficiency have become mandatory, under pain of […] Read More
Read MoreThe Apostille Is In!
Red-ribbon documents are now a thing of the past. Beginning May 14, 2019, the Philippine Government will now accept apostilled (or apostillized) documents as a replacement for legalization or consular authentication with the Philippine Embassy. May 14, 2019 is the official date when the Philippines accedes to the Apostille Convention. Beginning this date, the Philippines […] Read More
Read MoreThe New Social Security Act of 2018
Republic Act No. 11199, otherwise known as the Social Security Act of 2018, expressly repealed Republic Act No. 1161 (the old Social Security Law) and Republic Act No. 8282 (amending the old previous Social Security Law). Signed into law on 07 February 2019, the new charter of the Social Security System took effect on 05 […] Read More
Read MoreA Glance at Abuse of Dominant Position under Competition and Antitrust Laws
The term abuse of dominant position refers to anticompetitive business practices in which a dominant firm may engage in order to maintain or increase its position in the market.[i] Abuse of dominance or monopolization is punished under competition laws worldwide. In the US, Section 2 of the Sherman Act penalizes a person who shall monopolize, […] Read More
Read MoreImportant Dates for the 2019 Philippine Elections
The election fever has once more hit the Philippines as we brace ourselves for the May 13, 2019 elections. However, it is important to remember that certain acts are prohibited during certain times. Our excitement should not prevent us from acting with utmost cautions to prevent violation of election […] Read More
Read MorePhilippine Holidays for 2019
Pursuant to Proclamation No. 555, dated 15 August 2018, the Philippine holidays (both regular and special non-working days) for 2019 are as follows: 01 January 2019, Tuesday – New Year’s Day (regular holiday) 05 February 2019, Tuesday – Chinese New Year (Special non-working day) 25 February 2019, Monday – EDSA People Power Revolution Anniversary (Special […] Read More
Read MoreRegistration of Voters in the Philippines
Suffrage has been defined as the right to vote in political elections. However, it has a deeper meaning than merely exercising the right to vote. Suffrage includes the right to shape the future. After all, in voting, we determine our leaders who will either steer us to the shore of development or to the deep […] Read More
Read MoreSubstitution of Candidates in Philippine Elections
Candidates for elective offices can be substituted. Pursuant to Section 77 of Batas Pambansa Bilang 881, otherwise known as the Omnibus Election Code, substitution of candidates is allowed only in cases of death, withdrawal or disqualification of the original candidate. In cases of death or disqualification, the substitute may file his Certificate of Candidacy up […] Read More
Read MoreA Brief Primer on Petitions to Deny Due Course or Cancel a Certificate of Candidacy in Philippine Elections
The information provided by a candidate in his Certificate of Candidacy (COC) cannot be taken lightly. Should a candidate make false statements of a material representation in the COC, his COC may be cancelled or denied due course. Section 78 of Batas Pambansa Bilang 881, otherwise known as the Omnibus Election Code, provides the remedy […] Read More
Read MoreCertificates of Candidacies in Philippine Elections
The importance of a valid certificate of candidacy rests at the very core of the electoral process. (Bautista vs. Comelec, G.R. No. 154796, 23 October 2003) As such, a person with a cancelled certificate is no candidate at all. The evident purposes of the law in requiring the filling of certificates of candidacy and in […] Read More
Read MoreHow to Resign Properly in the Philippines
If an employee is unhappy with his work or would like to seek greener pastures, the normal recourse is to resign. In San Miguel Properties v. Gucaban (G.R. No. 153982, 18 July 2011), the Supreme Court had the occasion to define resignation as follows: “Resignation — the formal pronouncement or relinquishment of a position or […]- Read More
Infographic: Mga Dapat Mong Malaman Tungkol sa 13th Month Pay
Bilang isang corporate lawyer in the Philippines, isa sa mga madalas na itanong sa akin ng mga empleyado sa kumpanya ay ang tungkol sa kanilang 13th month pay. Karaniwan ng itinatanong ng mga empleyado, lalo na ang fresh graduates, ang tungkol dito. Ano ba ang 13th month pay? Lahat ba ay entitled makakuha nito? Magkano […]  Read More
Read MoreLost Title? Not a problem.
What do you do when your precious land titles are lost? In the Philippines, land titles are issued under the Torrens System. The Torrens system of registration governs the issuance of titles covering real properties in the Philippines. These include privately owned land of whatever kind – agricultural land, commercial land, industrial land, and other […] Read More
Read MoreEmployer’s SSS Condonation Program ends September 2019
The Social Security System (SSS) recently issued a reminder/notice to all employers of the current SSS Condonation Program and the fast approaching deadline, to avail of the program. Under the law, a penalty of three percent (3%) per month is assessed on unremitted contributions. This penalty continues to accrue and to compound, from the date […] Read More
Read MoreCartel Criminalization and Leniency Policies in the Philippines
Cartel Criminalization and Leniency Policies in the Philippines (13 June 2018) “Let me start with the obvious: cartel behavior (price-fixing, market allocation, and bid- rigging) is bad for consumers, bad for business, and bad for efficient markets generally. And let me be very clear: these cartels are the equivalent of theft by well-dressed thieves, […] Read More
Read MorePhilippine Competition Law at a Glance
Philippine Competition Law at a Glance (updated 06 March 2020) On 21 July 2015, Republic Act No. 10667, otherwise known as the Philippine Competition Act was finally enacted and became effective on 08 August 2015. However, a transitional period was provided by the law whereby an existing business structure, conduct, practice or any act that […] Read More
Read MoreHow to file a Trademark Infringement Case with the Philippine Intellectual Property Office
Blood, sweat and tears. All these you invested so your brand can establish its niche in the market. After years of hard work, your brand is now popular. You now have a regular clientele who patronize your products bearing your trademark. Then one day, you realize that your sales declined. You wonder why. Upon investigation, […] Read More
Read MoreThe Philippine Data Privacy Law and Regulations tell us, “Beware the Ides of March!”
“Beware the ides of March!” is from William Shakespeare’s famous tragedy, Julius Caesar. It was a warning uttered by a soothsayer to Julius Caesar, that he should stay home and be careful when March 15, the ides of March, comes. However, for entities covered by the Philippines’ Read More
Read MoreData Privacy in the Philippines
Information and communications technology play a vital role in nation-building and development of the country. In the information age, he who holds information holds power. From macro-economic perspective, the free flow of information is concededly vital to the growth of any nation, and key to the success of any business. With the power that follows […] Read More
Read MorePhilippine Holidays for 2018
The Philippine holidays (both regular and special non-working days) for 2018 pursuant to Proclamation No. 269 are as follows: 01 January 2018, Monday – New Year’s Day (regular holiday) 16 February 2018, Friday – Chinese New Year (Special non-working day) 25 February 2018, Sunday – EDSA People Power Revolution Anniversary (Special non-working […] Read More
Read MoreWhen Can a Policeman Arrest You?
When we watch the news, we oftentimes see a scenario whereby a victim is pointing at the accused which would prompt the policeman to immediately arrest the accused and detain him. In movies, we see the policeman knocking on a person’s door and arresting the accused with the oft-spoken phrase “Magpaliwanag ka na lang sa […] Read More
Read MoreFAQs on Search Warrants
When a policeman knocks on your door and wants to search your house, will you let him in? If you are standing in a street, can a policeman just search through your belongings? The answer is no, unless he is armed with a search warrant. No less than Art. III, Section 2 of the […] Read More
Read MoreAng Krimen na Pagtataksil sa Asawa
Ang pagtataksil sa asawa ay isang krimen sa Pilipinas. Ang pakikipag-relasyon nang isang kasal na lalaki o babae sa iba ay pinaparusahan nang ating batas. Hanggat walang hatol ang isang korte na pinapawalang bisa ang kasal, hindi malaya ang isang kasal na babae o lalaki na sumama sa iba. Ang adultery ay isang krimen […] Read More
Read MorePhilippine Bank Secrecy Law Simplified
The current banter between President Duterte and Senator Trillianes about bank secrecy waivers has piqued the interest of the public on the matter of bank secrecy. So, what is bank secrecy? Is it absolute? Are there any exceptions? On 09 September 1955, Republic Act No. 1405, otherwise known as An Act Prohibiting Disclosure of […] Read More
Read MoreThe Philippines’ Nationwide Smoking Ban under Executive Order No. 26: Separating Fact from Fiction
The “action-packed” presidential issuance from President Rodrigo Duterte, signed on 16 May 2017, Executive Order No. 26 (EO 26), otherwise known as the Nationwide Smoking Ban, takes effect sixty (60) days from its publication in a newspaper of general circulation. Thus, the smoking ban will be enforced throughout the Philippines beginning 23 July 2017. As […] Read More
Read MoreDawn Raids? No problem. The Rule on Administrative Search and Inspection under the Philippine Competition Act
Enforcement of the Philippine Competition Act Republic Act No. 10667, otherwise known as the Philippine Competition Act (“PCA”), generally prohibits three acts namely: anticompetitive agreements, abuse of dominant position and anticompetitive mergers and acquisitions. Pursuant to the law, the Philippine Competition Commission (“PCC”) is tasked with investigation, prosecution and adjudication of cases involving violations of the PCA. […] Read More
Read MoreThe Revised Regulations are Out! The 7 Commandments of Distraction-Free Driving
The June 2017 Revised Implementing Rules and Regulations (Revised IRR) for Republic Act No. 10913, also known as the Anti-Distracted Driving Act, is out, and will take effect fifteen (15) days from publication in either the Official Gazette or in any newspaper of general circulation. Here are what you should know about the Revised IRR, […] Read More
Read MoreHow to Get a License to Operate a Recruitment Agency in the Philippines
So you want to engage in the business of operating an employment agency deploying Filipino workers abroad? Don’t get too excited and commence business immediately. You must bear in mind that you cannot just recruit and deploy without a license from the Philippine Overseas Employment Administration (“POEA”). Otherwise, you run the risk of being criminally […] Read More
Read MoreOwnership of Land Can be Taxing! A Guide to Real Property Tax in the Philippines
Everyone dreams of owning their own house and lot. When you achieve such goal and receive your Transfer Certificate of Title, all the years of hard work have indeed paid off. However, being a landowner has its consequences. You now have to pay real property tax or “amelyar”. If you fail to do so, your […] Read More
Read MoreThe Invisible Yet Invincible Maritime Lien
As a general rule, when ownership of personal property (chattel), like a car for example, is transferred to another under a clean title – meaning, that no mortgage is annotated in the car’s registration – the transfer of ownership is complete. The creditors of the original owner cannot run after the car because the personal […] Read More
Read MoreCooking-up Your Contracts using “Boilerplates”
“Boilerplate” provisions refer to template provisions or “one-size-fits-all” clauses usually found at the end of most contracts. Because they are so-common, these provisions are often taken for granted especially by lawyers. To the perceptive eye however, boilerplate provisions should be considered as gems in a contract because they complete not only the aesthetic value, but […] Read More
Read MoreBasics on the Contract of Agency
We oftentimes hear the words “agency”, “principal”, “agency relationship” and “ahente”. So, what do these words mean? What is the relationship between an agent and his principal? What are their liabilities? Read on and learn the basics. The Civil Code of the Philippines is the governing law on agency. Article 1868 of the Civil Code […] Read More
Read MoreBasics on the Sale on Consignment
When you are engaged in retail trade, sometimes it is not practical to buy huge volumes of stocks to sell to your customers as this will require a higher cost on your part. You also subject yourself to the risk of not being able to sell all the products. Thus, an alternative to an outright […] Read More
Read MoreThe Nature of Sublease Contracts
Say for example you are renting a business space or apartment, not from the owner, but from another lessee. Under the law, you are considered as a sublessee. If the owner of the property tries to enforce the terms of his lease agreement with your lessor, what do you do? Should you respect the lease […] Read More
Read MoreFAQs on Martial Law in the Philippines
1. Who can place the Philippines under martial law? — The President of the Philippines can place the Philippines under martial law. 2. What areas of the Philippines can be placed under martial law? — Any part of the Philippines may be placed under martial law. 3. What law empowers the President to […] Read More
Read MoreFAQs on Having a Child Declared Legally Available for Adoption in the Philippines
1. What law governs the declaration of children as legally available for adoption? Republic Act No. 9523 (RA 9523) is the governing law. It requires the certification of the Department of Social Welfare and Development (DSWD) to declare a child legally available for adoption. It also amends certain provisions of RA 8552, otherwise known as […] Read More
Read MoreFAQs on Adoption in the Philippines
1. What law governs domestic adoption? — Republic Act No. 8552 , or the Domestic Adoption Act of 1998, as amended by Republic Act No. 9523, governs local adoption. 2. Who may adopt? — Sec. 7 of RA 8552 provides that the following may adopt: (a) Any Filipino citizen of legal age, in possession […] Read More
Read MoreThings You Wonder About Conjugal Property but were Afraid to Ask
There is no doubt that you love your husband or your wife. However, you sometimes wonder what properties you own exclusively that are beyond the reach of your spouse. Not that you don’t trust your wife. After all, almost every time, you give her all your wages. You just simply want to know for your […] Read More
Read MorePayment of the 13th Month Pay
In the Philippines, the sound of Christmas bells not only evokes the feeling of Christmas cheer but also signals for the payment of the 13th month pay for employees. Under Presidential Decree 851, all rank-and-file employees are entitled to the 13th month pay regardless of the amount of basic salary that they receive in […] Read More
Read MoreJoint Ventures in the Philippines
We can’t do everything by ourselves. Sometimes, we need a little help. Oftentimes, we need a joint venture. The Philippine Supreme Court has described a joint venture as an association of persons or companies jointly undertaking some commercial enterprise; generally, all contribute assets and share risks which requires a community of interest in […] Read More
Read MoreLicensing Agreements in the Philippines
Licensing agreements, including the assignment, licensing or transfer of intellectual property rights, must be recorded with the Philippine Intellectual Property Office (“IPO”) in order to be enforceable. However, these agreements need not be recorded if they conform with the requirements of the Intellectual Property Code of the Philippines as to mandatory clauses and prohibited clauses. […] Read More
Read MoreHow to Close a Corporation through the Shortening of its Corporate Term in the Philippines
Life is tough. So is business. So when the going gets tough, sometimes corporations need to close down. However, it is not as simple as packing up your things and leaving. A formal process must be done to legally close a corporation. One method is through the shortening of the corporate term. In the […] Read More
Read MorePayment of Night Shift Differential in the Philippines (updated last 10 June 2020)
Do you sometimes wonder why call center agents have salaries higher than some employees? One of the reasons is the night shift differential pay. Under Philippine labor laws, an employee who works between 10 p.m to 6:00 a.m. should be paid a night shift differential of not less than ten percent (10%) of his regular […] Read More
Read MoreHow to Appeal Trademark Cancellation and Opposition Cases in the Philippines
If you have lost in a trademark opposition or cancellation case (classified as inter partes proceedings) with the Philippine Intellectual Property Office, do not lose hope. The losing party has remedies under the law for the appeal of his case. Appeal to the Director of the Bureau of Legal Affairs A party wishing […] Read More
Read MorePhilippine Holidays for 2017
The Philippines is one of the countries with the most generous number of public holidays. It is very important for businessmen to know these holidays to ensure that they are paying the proper holiday pay to their employees. The two common types of holidays are regular holidays and special non-working days. Contrary to popular belief, […] Read More
Read MoreCreating a Company with a Foreign Principal in the Philippines (Updated as of January 2021)
Foreigners can do business in the Philippines. In fact, foreign investment contributes greatly to the Philippine economy. Some foreigners wish to establish companies in the Philippines which either form part of the company abroad or with a separate entity from its foreign principal. Either way, companies in the Philippines with foreign principals must be duly […] Read More
Read MoreHow to Cancel a Trademark in the Philippines
What will you do if a company is using your trademark? What if this company has fraudulently registered your trademark in his own name? The remedy to this problem is to have that company’s trademark cancelled. Section 1511.1 of the Intellectual Property Code of the Philippines (“IP Code”) provides: “151.1. A petition […] Read More
Read MoreAnnoying Someone is a Crime (updated last 10 June 2020)
Did you know that the act of annoying someone is a crime? Yes, it is and we certainly are not pulling your leg. The act of annoying someone is called unjust vexation and considered a form of light coercion punishable under Article 287 of the Revised Penal Code, to wit: “Art. 287. Light coercions. — Any […] Read More
Read MoreHow To Punish an Unfaithful Husband In The Philippines
Why do husbands cheat on their wives? We do not know the answer to that. However, we do know that cheating on one’s wife is punishable by law. Article 334 of the Revised Penal Code punishes the husband when he commits any of the following acts: 1. He shall keep a mistress in […] Read More
Read MoreChildren aboard Motorcycles
When plying streets in the Philippines, one can see the surge of motorcycles on the road. Sadly, there are irresponsible motorists who not only disregard traffic laws but also their own and their loved one’s safety. An example of such is the presence of small children aboard motorcycles. This practice poses a risk to the […] Read More
Read MoreBorn & Living in the Philippines but Not a Filipino? Here’s an easier way to become a naturalized Filipino Citizen
Were you born in the Philippines and living here all your life but still considered a foreigner? If you wish to become a Filipino citizen, then you can apply for administrative naturalization. Republic Act No. 9139 otherwise known as the Administrative Naturalization Law of 2000, was enacted as a remedial measure intended to make […] Read More
Read MoreAre you a Consumer? Then Read This: CHANGE IS COMING!
Yes, that is right. All consumers must be given the exact change by business establishments. The newly passed Republic Act No. 10909 mandate this in very clear terms. The law prohibits business establishments from giving insufficient or no change to consumers, and provides penalties ranging from Five Hundred Pesos (P500.00) or three percent of the […] Read More
Read MoreLifting Foreign Ownership Restrictions in the Philippines
Not long ago, the Philippines adhered to a protectionist policy, implementing numerous restrictions on foreign ownerships of businesses in the Philippines. In the past, the Philippine retail business was reserved exclusively for Filipino citizens. However, this changed with the legislation of the Trade Liberalization Act of 2000, now allowing the entry of foreign capital in […] Read More
Read MoreLove is made of Plastic: New Credit Card Regulations that You Should Know
They come in almost all colors: red, orange, gold (yellow), green, blue, indigo, and black. Some are in precious metals like titanium or platinum, or in rare gems like diamond, or sapphire. These little pieces of plastic can be your best friend in times of need, or your worst nightmare, in times of want. Whether […] Read More
Read MoreEmployers Beware: Age does not matter, unless you’re getting cheese
In the Philippines, it is now illegal to hire employees based on age. Long gone are the days when you will see an advertisement: “Wanted Secretary, 18-34 years old” or “Wanted driver, between 25-40 years old”. The practice of employers selecting employees because of age, or placing an age limitation as a qualification for employment, […] Read More
Read MoreAre You a Distracted Driver?
The crime of “Distracted Driving” is a new legal term. Punished under Republic Act No. 10913, which lapsed into law during the latter part of August 2016, a person caught running a vehicle with one hand, while operating any electronic communications equipment such as a mobile phone, two-way radio, tablet, hand-held computer, video player, gaming […] Read More
Read MoreA Simple Guide to Contract of Sale of Real Property
Selling property should not be a complex matter. Although it is advisable to secure the services of a lawyer to draft the Contract of Sale, there are templates that are downloadable in the internet. Of course, care must be taken in using these templates since they are not customized and may not be entirely applicable […] Read More
Read MorePunishing Violence Against Women in the Philippines
Republic Act No. RA 9262 , otherwise known as the Anti-Violence Against Women and Their Children Law (“VAWC”), defines violence against women and children as “any act or a series of acts against a woman who is his wife, former wife or against a woman with whom the person has or had sexual or dating […] Read More
Read MoreIntellectual Property Protection in the Philippines
The Philippines adheres to the Paris Convention for the Protection of Industrial Property Rights, the Berne Convention for the Protection of Literary and Artistic Works, the Patent Cooperation Treaty, the TRIPS Agreement and the WIPO Copyright Treaty, among others. In this light, intellectual property such as copyright, trademark, patent, utility model, industrial design are […] Read More
Read MoreHow to Close a Business in the Philippines
Financial reverses, shift to a different industry, migration to another country, incessant problems are just some of the reasons why some people close their business. Whatever the reason is, it is always a painful decision to raise your hands in defeat and give up on your business venture. Nevertheless, one cannot just pack up and […] Read More
Read MoreMaternity Rights in the Philippines
Motherhood is a gift and the ability of a woman to endure pregnancy is a miracle that has perplexed even the scientific minds. However, behind this wonderful gift is a painful reality that women worldwide have to endure in the workplace. Discrimination, lack of support and adherence to archaic chauvinistic policies have made it difficult […] Read More
Read MoreThe Philippine Competition Law: Regulating Mergers and Acquisitions, Monopolies and Combinations in Restraint of Trade in the Philippines
(updated 06 March 2020) Free trade in the Philippines has been a battle cry of businesses in all nations. With the influx of globalization, new business models have been created, leading to more innovative modes of doing business in the Philippines. On the face of a highly competitive business environment in the Philippines, profitable commercial entities […] Read More
Read MoreHow to Conduct Due Diligence in the Philippines
In this diverse society, it is inevitable that at one point in our lives, we will have to conclude or close deals with strangers. Although in an ideal world, trust should be sufficient, it is difficult, if not outright dangerous, to rely solely on good faith. Whether we are hiring an employee, buying land, […] Read More
Read MoreUnderstanding Property Relations Between Husband and Wife in the Philippines
“For richer, for poorer, in sickness or in health, to love and to cherish till death do us part.” This is part of the marriage vow being recited during weddings. Being married to someone entails not only sharing one’s life but also one’s property with the other. Hence, it is very important to know […] Read More
Read MoreQualifications for Philippine Elective Office
Qualifications prescribed by law for public elective positions in the Philippines are continuing requirements and must be possessed for the duration of the officer’s active tenure. It is the Philippine Congress which has the power to prescribe additional qualifications and disqualifications. The following is a simple list of the pertinent qualifications required for public elective […] Read More
Read MoreUnderstanding Statutory Leaves of Employees in the Philippines
As the old saying goes, all work and no play makes Jack a dull boy. Hence, part of improving an employee’s efficiency is to allow him to take a leave from work without sacrificing his income. The Philippine Labor Code and other special laws mandate certain leaves that an employer should extend to an […] Read More
Read MoreLimitations on Foreign Investments and Equity in the Philippines
Foreign investments are encouraged by the Philippine government to fuel economic growth. Based on data culled from the National Statistical Coordination Board (“NSCB”), the total approved foreign investments in the Philippines for the year 2014 amounted to P186.9 Billion Pesos (or roughly US$4 Billion Dollars). According to the NSCB, in 2014, Japan is the largest […] Read More
Read MoreBenefits of Getting a Trademark Agent
If you are interested in filing a trademark application in the Philippines or wish to protect your business brand or trademark, it is quite tempting to undertake the task on your own. As a businessman, saving on costs is a primary concern. Consequently, most businessmen fall into the […] Read More
Read MoreMaintaining Your Trademark in the Philippines
The Philippine Intellectual Property Office (“IPO”) does not require proof of use in commerce in the processing of trademark applications. However, during the life of the trademark, the applicant or registrant must prove that it is using the mark in the Philippines. Thus, a notarized Declaration of Actual Use (“DAU”) […] Read More
Read MoreTrademark Opposition Proceedings in the Philippines
A party may file an opposition to a Philippine trademark within a period of thirty (30) days from the date of publication of the mark in the e-gazette of the Philippine Intellectual Property Office (IPO). The e-gazette may be viewed at www.ipophil.gov.ph . The proceedings are initiated by the filing of the verified notice […] Read More
Read MoreThe Treatment of Medical Malpractice in the Philippines
Proving medical malpractice in the Philippines one of the more difficult civil cases in the Philippines. Medical procedures involve, to a great degree, technical matters, which must be clearly understood first, prior to pursuing a claim that a treatment was attended with malpractice. Moreover, in order to prove the existence of medical malpractice in any […] Read More
Read MoreHow to Compel a Corporation to Grant Access to Corporate Records
Access to corporate records is vital in any corporation. Whether these records pertain to financial documents, human resources, operation endeavors, future plans, or even building and floor plans, these records help the officers, directors, and the stockholders map out the present and future directions for the company. In view of their significance, access to the […] Read More
Read MoreHow to Implement a Redundancy Program in the Work Place
Dismissal of workers due to redundancy is a management prerogative governed by the Article 283 of the Labor Code. This is one of the authorized causes which an employer, in good faith, may utilize as a measure of efficiency in the company, or to prevent company losses. The pertinent portions of the law provide: Article […] Read More
Read MoreHow to Transfer Shares of Stock in a Corporation
Philippine law treats shares of stock in a corporation as personal property. Similar to other personalty, the owner of the property can sell, assign, transfer or convey his property to another as he wishes. This is an attribute and principle of ownership which cannot be taken away. However, being in the nature of intangible personal […] Read More
Read MoreWhen is an Employee Entitled to Separation Pay?
Separation pay has been defined as the amount that an employee receives at the time of his severance and is designed to provide the employee with the wherewithal during the period he is looking for another employment. However, this broad definition must not be taken at face value. There are only limited instances wherein separation […] Read More
Read MoreHow to Do Business Legally in the Philippines
Contrary to popular belief, it is actually easy to legally do business in the Philippines. The Philippine government has taken great strides to ensure ease of doing business in the country. You must first remember that in order to do business in the Philippines, you must choose the business vehicle that you wish to utilize. […] Read More
Read MoreHow to Get a Special Investor’s Resident Visa (SIRV)
How to Get a Special Investor’s Resident Visa (SIRV) The Special Investor’s Resident Visa (SIRV) is a visa issued by the Bureau of Immigration through the Board of Investments (“BOI”) pursuant to the provisions of the Omnibus Investments Code of 1987. The SIRV is a special non-immigrant visa that entitles the holder to reside in […] Read More
Read MoreIllegal recruitment and its Penalty
What is illegal recruitment? Republic Act No. 10022, otherwise known as the Migrant Workers Act” defines illegal recruitment as any act of canvassing, enlisting, contracting, transporting, utilizing, hiring, or procuring workers and includes referring, contract services, promising or advertising for employment abroad, whether for profit or not, when undertaken by non-licensee or non-holder of authority. […] Read More
Read MorePhilippine Representative Office
A Representative or liaison office deals directly, with the clients of the foreign parent company but does NOT derive income from the Philippines and is fully subsidized by its foreign head office. It undertakes activities such as but not limited to information dissemination and promotion of the company’s products as well as quality control of […] Read More
Read MorePhilippine Branch Office
A Branch office of a foreign company carries out the business activities of the foreign head office and derives income from the Philippines. It has NO separate legal personality since it is merely an extension of the foreign head office. The minimum inward remittance for a branch office is Two Hundred Thousand United States Dollars […] Read More
Read MorePhilippine Subsidiary
Should you wish to incorporate a Philippine subsidiary, you need to file an application with the Securities & Exchange Commission. It must be noted that a subsidiary has a distinct and separate personality from its parent company. In forming a subsidiary, it must be borne in mind that the Corporate Secretary and Corporate Treasurer must […] Read More
Read MoreSlander & Oral Defamation
Being the subject of unfair criticism and gossip are matters which adversely affect persons from all walks of life. At one point, we have encountered situations wherein we have been victimized by insulting and defamatory remarks. Oftentimes, we ignore such hurtful and unfounded insinuations. However, when things get out of hand and our reputation is […] Read More
Read MorePhilippine Estate Tax
IMPORTANT: This article is relevant only to those who died before 01 January 2018 since Republic Act No. 10963, otherwise known as the Tax Reform for Acceleration and Inclusion Law (TRAIN Law), amended the Tax Code, including the procedure, tax rates and deductions for estate taxes. The TRAIN Law became effective on 01 January 2018. […] Read More
Read MoreHow To Get Business Permits in the Philippines
Before commencing your business in the Philippines, you need to secure a Business permit or Mayor’s permit, as it is commonly called, from your local government where your business is located. Here are some notes on business permits: • Business permits expire on the 31st of December unless the permits are issued on a quarterly […] Read More
Read MoreHow to Get a Trademark in the Philippines
Trademark applications are filed with the Philippine Intellectual Property Office (IPO). Filipinos or Filipino corporations may directly file their applications with the IPO. However, foreigners or foreign corporations have to appoint a local agent or representative in order to file applications with the IPO. Applying for a trademark is not like getting a business permit […] Read More
Read MoreHow to Get an Alien Employment Permit in the Philippines
Before a foreigner commences work in the Philippines, he must first secure an Alien Employment Permit (AEP) from the Department of Labor and Employment (DOLE). However, not all foreigners are required to do so. The following are exempted from securing an AEP: 1. Members of the diplomatic services and foreign government officials accredited by the […] Read More
Read MoreHow to Apply for Certification as a Barangay Micro Business Enterprise (BMBE)
Any person, natural or juridical, cooperative or association, or business entity or enterprise engaged in production, processing, manufacturing of products or commodities, including agro-processing, trading and services may apply for Certificate of Authority as a BMBE provided that they should have at least a total asset value, including those arising from loans but exclusive of […] Read More
Read MoreHow To Avail of Leave for Victims under the Anti-Violence against Women Law
Women employees who are victims as defined in Republic Act No. 9262, otherwise knows as the Anti-Violence Against Women and Their Children Law, are entitled to a leave of up to ten (10) days with full pay. The said leave shall be extended when the need arises, as specified in the protection order issued by […] Read More
Read MoreHow To Terminate An Employee Based on Just Causes
An employee may only be terminated for just causes or for authorized causes under the Labor Code. The following are considered as just causes: 1. Serious misconduct or willful disobedience by the employee of the lawful orders of his employer or representative in connection with his work; 2. Gross and habitual neglect by the employee […] Read More
Read MoreHow To Place an Employee Under Preventive Suspension Pending Investigation
Suspension of an employee may either be 1) imposed as an administrative penalty for infractions committed or 2) preventive suspension pending investigation of an employee. If we are dealing with the second kind (preventive suspension pending investigation), the following conditions must be met: a. The employer may place the worker concerned under preventive suspension if […] Read More
Read MoreHow To Avail of Parental Leave for a Solo Parent
Republic Act No. 8972 grants parental leave of seven (7) work days with full pay every year, in addition to leave privileges under existing laws, to solo parents. Parental leave for solo parents is granted to any solo parent or individual who is left alone with the responsibility of parenthood due to: 1. Giving birth […] Read More
Read MoreHow To Register a Sole Proprietorship with the DTI
Businesses can be conducted in many ways. It may be through a sole proprietorship, partnership or corporation. Sole proprietors must register with the Department of Trade & Industry (DTI) while corporations and partnerships are registered with the Securities & Exchange Commission (SEC). A sole proprietor must register with the DTI and secure a Certificate of […] Read More
Read MoreHow To Get a Special Resident Retiree’s Visa (SRRV)
The Special Resident Retiree’s Visa (“SRRV”) is a visa issued by the Bureau of Immigration through the Philippine Retirement Authority (“PRA”). The SRRV is a special non-immigrant visa that provides its holders with multiple-entry privileges and indefinite stay in the Philippines and is especially designed for those who wish to live in the Philippines on […] Read More
Read MoreShrink-Wrap Agreements: Are They Valid Contracts?
A person who purchases a cellphone, portable dvd player, a coffee maker, or toy finds warranty cards and other written contract terms inside the box. If the product is contained in a cellophane-wrapped box, sealed, with tamper-proof packaging, these terms are available for review only after the product has been bought and then opened, in […] Read More
Read MoreAre Contractual Employees Entitled to Separation Pay?
A fixed-term employee or contractual employee is a type of employee whose employment is fixed for a certain period of period of time. When the contract expires and is not renewed by his or her employer, the employment of the contractual employee is deemed to have automatically terminated. It is of common knowledge that the […] Read More
Read MoreDe-Mystifying the Bouncing Checks Law (B.P. 22)
A common predicament faced by businessmen is violating the Batas Pambansa Blg. 22 also known as the Bouncing Checks Law. Evidently, businessmen issue checks as a matter of practice, and sometimes when the due dates of these checks fall, either by inadvertence or unavailable finances, the check bounces. BP 22 punishes a person for issuing […] Read More
Read MoreProtecting your Intellectual Property Rights
The protection of intellectual property rights is a primordial concern, especially in the field of business format franchising. Business format franchising, to a large extent, involves the use by a person called a franchisee of the intellectual property another called the franchisor, of course, with the consent of the latter. The intellectual property involved in […] Read More
Read MoreLawyers’ Fees: Types of Legal Fee Arrangements
In “shopping” for a lawyer, the first thing the usually comes to mind is how much a lawyer charges. Generally, the professional fees of a lawyer depend on the nature and complexity of the case, the financial capacity of the client, the extent of work required, among other factors. The usual legal fee arrangements are: […] Read More
Read MoreBringing in Foreign Investors in Retail Trade
You have a business idea in mind; a great concept which you think will be very profitable. You have the over-all know-how to accomplish the business, and the will to succeed. You need capital and have been thinking of bringing in foreign investors to the scene. This is a common-place scenario in business nowadays. The […] Read More
Read MoreAn Entrepreneur’s Guide to Employment Contracts
An employment contract is an essential part of every business. An incomplete or badly drafted employment contract could spell trouble to an employer from both a legal and business perspective. For instance, if an employee learns of certain trade or business secrets of an employer during employment, an unguarded employment contract may mean that when […] Read More
Read MoreThe Separate Personality of a Corporation
A corporation is defined by the Corporation Code as an artificial being created by operation of law, having the right of succession and the powers, attributes and properties expressly authorized by law or incident to its existence. Consequently, as an artificial being, a corporation has a personality separate and distinct from its stockholders. Hence, the […] Read More
Read MoreDissecting a Franchise Agreement: Part One
If you are interested in franchising, whether as a franchisor or a franchisee, an important document to consider is the franchise agreement. A franchise agreement is usually a voluminous document. Of course, it is not the intention of the franchisor to confuse the franchisee. It is lengthy because it specifies in detail what the respective […] Read More
Read MoreDissecting a Franchise Agreement: Part Two
The first part of “Dissecting a Franchise Agreement” dwelled on the subject matter of the franchise agreement, namely, the business format being franchised. Since a franchise agreement is usually a lengthy document, it is best that the agreement be broken down into its basic elements, to allow prospective franchisors and franchisees to understand its bare […] Read More
Read MoreDissecting a Franchise Agreement: Part Three
The last element of any contract, which is necessarily present in a Franchise Agreement, is the element of cause or consideration. Our law defines cause or consideration as the prestation or promise of a thing or service by the other. In a Franchise Agreement, the cause or consideration involves the “why?” in the agreement. Why […] Read More
Read MoreDissecting a Franchise Agreement: Part Four
We have dissected the Franchise Agreement down to its most basic elements, namely: consent, subject matter, and cause or consideration. The first two parts of ‘Dissecting a Franchise Agreement’ discussed the two essential elements of a valid Franchise Agreement, namely, consent and subject matter. Part three focused on the cause or consideration which impels a […] Read More
Read MoreSole Proprietorship, Corporation or Partnership: Choosing your business enterprise
In doing business, there are various forms of business entities to consider, the most common forms of which are 1) single proprietorships, 2) corporations, and c) partnerships. A single proprietorship is a business operated by a single individual. Registration of single proprietorships would take between 1-2 days, and the documentary requirements of the Department of […] Read More
Read MoreAnnulment of Marriage in the Philippines
There is no divorce in the Philippines. However, a marriage may be annulled in the Philippines for any of the following causes, existing at the time of the marriage: (1) Either party was eighteen years of age or over but below twenty-one, and the marriage was solemnized without the consent of the parents, UNLESS after […] Read More
Read MoreSelf-Defense
Not all acts constitute crimes. A person who is defending himself, family or property cannot be held criminally liable. Article 11 of the Revised Penal provides for justifying circumstances whereby the person is said to be acting in accordance with law such as self-defense Art. 11. Justifying circumstances. — The following do not incur any […] Read More
Read MoreBattered Woman Syndrome
In criminal and family law, the novel concept of Battered Woman Syndrome (BWS) vis-à-vis self-defense has been a controversial topic. Republic Act No. 9262, otherwise known as the Anti-Violence Against Women and Their Children Act (“VAWC”) puts into law this concept and states: SECTION 26. Battered Woman Syndrome as a Defense. – Victim-survivors who are […] Read More
Read MoreHow to Form a Partnership in the Philippines
If you are interested in engaging in business or practicing your profession with some colleagues, a partnership may be the proper vehicle. The Philippine Civil Code provides for a definition of a partnership as follows: Art. 1767. By the contract of partnership two or more persons bind themselves to contribute money, property, or industry to […] Read More
Read MoreEmployee Benefits under Philippine Laws
Listed below is a summary of the mandatory benefits and provisions for employees in the Philippines under the Labor Code and special laws: 1. Minimum wage = P537.00 per day (in NCR) as of 22 November 2018 2. 13th month pay (after 1 month of service) = 1/12 of the total basic salary earned by […] Read More
Read MoreHow to Get a Patent
When through your own efforts or resources, you subsequently develop an invention, new technology or breakthrough, it is important to have it protected. Such intellectual property can be copied or exploited by others if it is not protected. The usual form of protection is to apply for a patent with the Bureau of Patents of […] Read More
Read MoreLifting the Ban of Night Work for Women and Expanding the Rights of Night Workers
Under the old Labor Code, women were prohibited from engaging in night work. As such, Article 130 of the Labor Code provides: Art. 130. Nightwork prohibition. No woman, regardless of age, shall be employed or permitted or suffered to work, with or without compensation: a. In any industrial undertaking or branch thereof between ten o’clock […] Read More
Read MoreSexual Harassment
Republic Act No. 7877, or the Anti-Sexual Harassment Act of 1995 (RA 7877), is the governing law for work, education or training-related sexual harassment. RA 7877 states that “work, education or training-related sexual harassment is committed by an employer, employee, manager, supervisor, agent of the employer, teacher, instructor, professor, coach, trainor, or any other person […] Read More
Read MoreHow to Apply for a US Tourist Visa
Applying for a US tourist visa is not as burdensome as it was before. Gone are the days when one has to camp outside of the US Embassy just to apply for a visa. At present, the US Embassy has employed a systematic appointment system. The usual types of US visa are the B-1 and […] Read More
Read MoreHow to Amend the Articles of Incorporation
The Articles of Incorporation is the bible of a corporation. It spells out the pertinent information concerning: a. The name of the Corporation b. The primary and secondary purposes of the Corporation c. Place of principal office of the Corporation d. Term of existence of the Corporation (normally 50 years) e. Names, nationalities, and residences […] Read More
Read MoreImproving Efficiency in the Philippine Government – The Anti-Red Tape Law
Entrepreneurs are exposed to government agencies in the usual daily grind of their business. Most companies have to submit monthly forms to the Bureau of Internal Revenue such as the 2550M (Monthly Value-Added Tax Declaration), or the 2551 M (Monthly Percentage Tax Return) or the 1601-C (Monthly Remittance Return of Income Taxes Withheld on Compensation). […] Read More
Read MoreExtrajudicial Settlement of Estate in the Philippines
Settlement of an estate need not undergo judicial proceedings all the time. Rule 74, Section 1 of the Rules of Court allows the extrajudicial settlement of estate by agreement among the heirs. Said Rule states: Sec. 1. Extrajudicial settlement by agreement between heirs. – If the decedent left no will and no debts and the […] Read More
Read MoreHow to Sell your Business
Selling your business can be motivated by many factors such as inability to continue the business, business losses, buy-out by a competitor, handsome offer by an investor, to name a few. The decision to sell your business will depend upon your sound business judgment and forecasts on the movement of the market. Whatever reason impels […] Read More
Read MoreDO No. 18-A: Strengthening the Rights of Contractual Employees & Imposing Obligations on the Contractors
Contracting and sub-contracting arrangements are commonplace in most business transactions. Instead of hiring their own messengers, janitors and security guards, among others, entrepreneurs have learned the value of outsourcing these services to contractors. Truth be told, contracting out these jobs is actually more cost-efficient in terms of time and money for the usual businessman. However, […] Read More
Read MorePhilippine Holidays for 2012
On 24 November 2011, the President issued Proclamation No. 295 which declares the regular holidays, special non-working days and special holidays for the year 2012. Employers and employees alike should be familiar with these days. On the side of the employee, he needs to know the days when he is not required to work while […] Read More
Read MorePhilippine Copyright
Republic Act No. 8293, otherwise known as the Intellectual Property Code of the Philippines (“IP Code”), is the law governing copyright, in addition to international treaties which the Philippines is a signatory to. Under the IP Code, copyright subsists in original and intellectual creations which are defined by the enumeration of such works namely: 1. […] Read More
Read MoreCopyright Infringement
Copying original and intellectual creations is considered as copyright infringement and is punishable under Republic Act No. 8293, otherwise known as the Intellectual Property Code of the Philippines (“IP Code”). In order to prove copyright infringement, one must show that he has a valid copyright in the work allegedly infringed and that the perpetrator infringed […] Read More
Read MorePhilippine Industrial Design
The Intellectual Property Code of the Philippines defines an industrial design as any composition of lines or colors or any three-dimensional form, whether or not associated with lines or colors; provided that such composition or form gives a special appearance to and can serve as pattern for an industrial product or handicraft. Simply put, it […] Read More
Read MorePhilippine Utility Model
An invention patent is concerned with any technical solution of a problem in any field of human activity which is new, involves an inventive step and is industrially applicable. Such definition makes it difficult for people to secure an invention patent because most matters or products have already been invented. One may wonder what type […] Read More
Read MoreHow to Correct Errors in Your Birth Certificate without Going to Court
As a general rule, no entry in the civil register shall be changed or corrected without a judicial order. Hence, the usual process for correcting errors in the birth certificate is to file a petition in court. Fortunately, Republic Act No. 9048, as recently amended by Republic Act No. 10172, allowed the administrative correction of […] Read More
Read MoreOpposing a Trademark in the Philippines
If someone is seeking to register a trademark that may be confusingly similar to your trademark, you may file an opposition to such trademark within 30 days during its publication in the IPO e-gazette. Marks are published in the website www.ipophil.gov.ph. The Regulations on Inter Partes Proceedings govern petitions for opposition filed with the Philippine Intellectual […] Read More
Read MoreHoliday Pay in the Philippines
Holiday pay has been defined as payment of the regular daily wage for any unworked regular holiday. However, not all employees are entitled to holiday pay. The benefit does not apply to: 1. Government employees 2. Those of retail and service establishments regularly employing less than ten (10) workers; 3. Househelpers and persons in the […] Read More
Read MoreHow to Terminate an Employee on the Ground of Retrenchment
The right of management to dismiss workers on the ground of retrenchment to prevent serious losses is governed by Article 283 of the Labor Code. This is one of the authorized causes of termination of employment. However, before one can dismiss employees on the ground of retrenchment, four elements must be proven. Firstly, the losses […] Read More
Read MorePhilippine Labor Law: Types of Employees
In any business venture, there are two resources which must be considered: capital and labor. Every businessman knows the saying “to make money, you have to spend money.” Every business entails a bit of risk-taking. The ability and the willingness to take certain calculated risks are almost always essential for every business venture to succeed. […] Read More
Read MoreHow to File a Criminal Case in the Philippines
If you are a victim of crime or felony in the Philippines, it is wise to report the crime or felony with the barangay and police authorities. Thereafter, you must secure a barangay blotter and police blotter or report so that you may use them as evidences. If you sustained injuries, go to a government […] Read More
Read MoreHours of Work of Employees
HOURS OF WORK OF EMPLOYEES Article 83 of the Labor Code enunciates that the normal hours of work of any employee shall not exceed eight (8) hours a day. This is exclusive of the one (1) hour lunch break. The Supreme Court explained the rationale of this provision to safeguard the welfare of employees and […] Read More
Read MoreBatas Kasambahay – Law Governing Employers and Their Househelp or Domestic Workers
Republic Act No. 10381 or the “Batas Kasambahay” is the law governing the rights and liabilities of both the househelps or domestic workers and their respective employers or “amo”. The Batas Kasambahay requires an Employment Contract be executed between the domestic worker and the employer before the commencement of the service in a language or […]- Read More
Philippine Legal Advice
Learn more about legal rights and remedies in the Philippines by clicking on the corresponding links below. The information provided here should not be construed as legal advice or opinion. This is for information purposes only and is not intended to solicit or create, and does not create, an attorney-client relationship between NDV Law and […]